This article, written by Sabah Bharde, was a runner up for our Pharmacology Matters Writing Competition for early career pharmacologists in 2020.
The perception of pain is universal, with 6–7% of the global population suffering from debilitating, intense pain on a regular basis. The pursuit to treat pain has led us to natural agents. These natural pain-relieving compounds have enticed pharmacologists over centuries and include anti-inflammatory drugs such as aspirin (from the willow bark tree ‘Salix alba’) and opioids (obtained from the opium poppy ‘Papaver somniferum’).
To this day, opioids remain the gold-standard of analgesics in the clinic. Opioids can mimic actions of the body’s natural pain-relieving pathways and alter nerve activity throughout the nervous system, particularly in places where other analgesics are not able to work, making them powerful pain-alleviating drugs. But even these potent analgesics have mixed success, not to mention a risk of dependence and addiction. With a huge unmet need for new pain relief options, nature has steered scientists towards one protein which has great potential for the future for analgesic drug development.
At the turn of the century, UK researchers John Wood and Geoff Woods identified families in Pakistan that appeared unable to perceive pain. They discovered that mutations in the gene encoding a sodium channel (Nav1.7) were responsible for this lack of pain awareness, a condition termed Congenital Insensitivity to Pain (CIP). The Nav1.7 protein is a sodium channel found on the surface of pain-sensing neurones and acts like a gate, opening in response to painful stimuli. Wood and Woods used mice which had Nav1.7 in pain-sensing neurones removed by genetic engineering, to reproduce the pain-free phenotype. Nav1.7 has since been an attractive candidate for the development of new, non-opioid analgesic drugs. Until recently, it was unclear why efforts by drug companies had floundered, but a recent report by Wood showed that analgesia conferred by congenital Nav1.7 mutations rely on boosting the body’s natural pain-relieving system, the endogenous opioid system (Figure 1).

Avoiding physical harm is a universal evolutionary mechanism for survival, so it is unsurprising that modern molecular insights of pain mechanisms show parallels across animal species. The animal kingdom has been fundamental to our current understanding of human pain, particularly through investigating deadly toxins. For example, tetrodotoxin (TTX) is a notorious neurotoxin found in fugu or puffer fish and is deemed the most delicious fish in Japan. TTX owes its lethal effects to the fact it blocks sodium channels, including Nav1.7, which halts all nerve signals, including pain signals.
Spiders may also harbour a reservoir of novel painkillers. A tarantula-inspired synthetic peptide, protoxin II (ProTx-II), originally isolated from Peruvian green velvet tarantula (Thrixopelma pruriens) venom, made rats insensitive to pain by blocking Nav1.7. It is known to be one of the most potent Nav1.7 blockers found yet, but its attraction to other types of sodium channels means it can cause side effects like heart failure and paralysis. In 2017, Pn3a, a peptide from the giant blue bloom tarantula (Pamphobeteus nigricolor), inhibited Nav1.7 activity with 1000-fold selectivity over all other types of sodium channels. But the venom was found to only serve as an analgesic in rodents when given with a small dose of opioids.
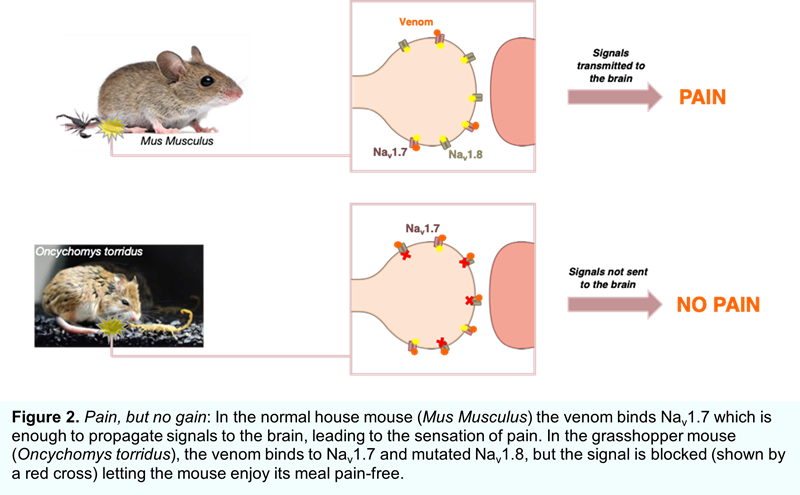
Scorpions are known to have a reputation for the most deadly attacks in the animal kingdom. The bark scorpion (Centruroides sculpturatus) causes an intensely painful and potentially lethal sting that deters snakes and other predators. But the grasshopper mouse (Oncychomys torridus), distantly related to the ordinary house mouse (Mus musculus), is completely resistant to pain produced by the scorpion sting. In fact, the grasshopper mouse even preys upon scorpions! The grasshopper mouse’s resistance to bark scorpion stings makes for a fascinating study and has implications for developing pain relieving drugs for humans. Evolutionary neurobiologist Ashlee Rowe and colleagues first reported the endurance of the grasshopper mouse to scorpion stings in 2013, where they suspected a mutation in Nav1.7. They later found that this channel functioned normally in response to the venom, and instead another sodium channel (Nav1.8) was responsible (Figure 2). Normally, once Nav1.7 initiates the pain signal, its counterpart Nav1.8 sustains it, but rather than refining Nav1.7 to spurn the venom, the grasshopper mouse has mutations in Nav1.8 that switches the channel off in response. The Nav1.8-block produced by the venom blunts signals triggered by other painful stimuli too – when the irritant formalin was injected into the paws of grasshopper mice, they were less agitated after a dose of scorpion venom − essentially turning the venom into a painkiller. The interactions between Nav1.8 and venom peptides are under investigation, in hope of designing molecules that will work in humans.
For over a decade, sophisticated technologies have expanded pain research but as yet, the only toxin-derived analgesic approved by the US Food and Drug Administration (FDA) is ziconotide (Prialt), a South Pacific cone snail (Conus magus)-based compound which targets calcium channels and is considered 1,000 times more potent than morphine. As this sea-dwelling analgesic also lacks morphine’s addictive potential, it has since been effectively used to treat patients that have severe chronic pain. Ziconotide serves as a forerunner molecule for proving that animal toxins can enter the market as drugs, but while the world's venomous creatures’ dwell in rapidly fading habitats, this serves yet another reason to better protect the natural world.
The preeminent discoveries guided by nature underpinned Nav1.7 as an important component of pain perception, and its analgesic synergy with endogenous opioid signalling highlights the intricacy of the pain system. These secrets of nature have already given useful insights into how we feel pain and will continue to interest researchers as they strive to discover new, powerful analgesics.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.