This article is a runner-up in the 2023 ECP writing competition. Megan Jackson’s piece on apathy engaged the judging panel, who liked Megan’s style of writing, and the topic that she chose.
You’ve probably heard the term apathy before. An image of a glassy-eyed teenager sprawled on their bed, scrolling on their phone may come to mind. Or perhaps you see it in yourself, when you’d rather chill out in front of the TV than get up and finish that task you’ve been putting off. The reality is, apathy is more than just a synonym for ‘can’t be bothered’, it’s a complex neuropsychiatric syndrome, with devastating health implications for those suffering from it. It is particularly common in the aged population, and those with neurodegenerative diseases such as Alzheimer’s and Parkinson’s Disease.
Apathy first emerged in the scientific literature to describe a phenomenon known as “prisoner of war syndrome” back in 1956. However, our understanding of what we know as apathy and how it affects different patient populations has rapidly expanded in recent decades. Together, the work of Marin, Levy and Dubois conceptualised apathy as a quantifiable construct with multiple domains of disrupted processing (behavioural, emotional and cognitive) which could be assessed via clinical questionnaires and even mapped onto distinct brain regions. To be diagnosed with clinical apathy, the patient has to show symptoms relating to at least two of these domains, for a period of more than four weeks.
It’s fair to say that apathy syndrome certainly has a ‘can’t be bothered’ element to it. It’s described as a motivational deficit, or quantitative reduction in goal-directed behaviour. This means the patient struggles to self-initiate a task, perform it with the same vigour they once did, and see it through to the end. Apathy can also affect how we process emotional information, so that our responses to both positive and negative stimuli become blunted. This means the patient may no longer react to a really exciting or really worrying piece of news in the same way they did before. Hobbies they once enjoyed, and friends they loved to see may no longer seem interesting. It’s therefore unsurprising that this has a profound impact on quality of life, both for the patient and for their caregivers. It’s also associated with increased mortality, as the patient stops taking care of themselves, taking medication, or seeking medical help when they need it. Clearly, apathy represents a significant clinical challenge, but surprisingly there is currently no agreed treatment approach for apathy syndrome.
As you read this, you may be thinking that apathy sounds a whole lot like major depressive disorder (MDD), and treatment with currently available antidepressants should help solve the problem. However, there is emerging evidence to suggest that depression and apathy are distinct constructs, with different underlying neurobiological mechanisms and treatment approaches. Brain imaging studies in patients with Parkinson’s Disease have shown that apathy and depression may involve different brain regions. It has been shown that treatment of apathy with antidepressants has little/no clinical effect, and in fact, could end up making symptoms even worse. Use of selective serotonin reuptake inhibitors (SSRIs), a first-line treatment for depression, can induce apathy syndrome, known colloquially as SSRI-induced indifference. Supporting this, a recent study by researchers at the University of Cambridge found that the commonly prescribed SSRI citalopram impairs performance in a task that measures emotion-based cognitive function in healthy volunteers. Despite all of this evidence, apathy and MDD are still often treated as the same thing, and SSRIs continue to get prescribed to apathetic patients.
Part of the reason for treating apathy and depression as the same thing is driven by difficulties in distinguishing them clinically. They certainly share overlapping characteristics, particularly loss of motivation and engagement with daily activities. However, studies looking closer at data from commonly used clinical apathy and depression questionnaires found that although they do align in the motivational deficit domain, they are dissociable in the emotional domain. While patients with depression may experience low mood, patients with apathy experience an emotionally blunted state. When questions relating to motivation are removed from the questionnaires, the correlation between apathy and depression disappears.
Given these key differences, it is clear that apathy syndrome requires a different treatment approach. Promisingly, there are potential treatments for apathy syndrome emerging in clinical trials. We know that motivational deficit is a key aspect of apathy, and there is a well-defined role of the mesolimbic dopaminergic (DA) system in the process of motivated behaviour. Apathy often occurs in diseases associated with DA loss, such as Parkinson’s disease and Huntington’s disease. With this in mind, tackling a potential reduction in DA transmission might have some clinical benefit. Methylphenidate, a drug which prevents the reuptake of DA had some clinical benefit on apathy scores in Alzheimer's disease in multiple randomised control trials. However, increasing DA levels doesn’t always seem to be the simple answer. Looking beyond the DA system, the cholinesterase inhibitors donepezil and rivastigmine, and the NMDA receptor antagonist memantine have also been shown to improve apathy scores. Interestingly, each of these drugs target distinct neurochemical systems (figure 1). How can drugs with such diverse mechanisms of action all help the same syndrome? Unfortunately, it’s very difficult to answer this question as we don’t know enough about the underlying neurobiology of apathy. Apathy is rarely studied in its own right. It is often studied as a symptom or associated syndrome in the context of another disease such as AD or PD, making it really difficult to figure out whether these treatments are targeting systems involved in apathy, or are treating the disease it’s associated with.
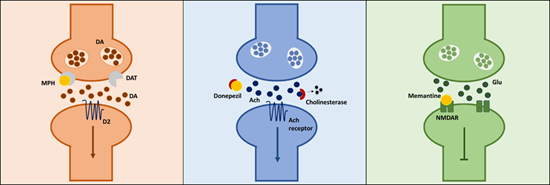
Fig.1. Simplified mechanistic pathways of methylphenidate, donepezil and memantine.
Methylphenidate prevents reuptake of synaptic DA by binding to DAT, thereby increasing amount of DA in the synaptic cleft. Donepezil binds to cholinesterase, preventing it from breaking down Ach, thereby increasing amount of Ach in the synaptic cleft. Memantine binds to the NMDAR, thereby blocking the action of glutamate at this receptor. MPH- methylphenidate, DA- dopamine, DAT- dopamine transporter, Ach – acetylcholine, Glu- glutamate, NMDAR - N-methyl-D-aspartate receptor.
A greater understanding of the mechanisms of apathy could help us understand how these treatments are working, and help develop new, more effective ones. This can be achieved best at the preclinical level by developing rodent models of apathy syndrome to look more closely at the behaviour and the mechanisms that drive it. Rodent models have been valuable in helping us find treatments for a wide range of diseases, and have made many new discoveries possible. However, the question must be asked: if it’s tricky to distinguish apathy and depression in humans, how would you go about doing it in rodents? A recent review suggests how apathy and depression-related behaviour can be distinguished at the preclinical level, to the benefit of drug development.
By taking the time to distiguish apathy and depression at the patient and preclinical level, we can more meaningfully test the potential of novel therapeutics, and help develop an agreed treatment approach for patients with apathy syndrome.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.