This piece by Marcus Ilg is a runner-up for our 2023 ECP writing competition. Judges enjoyed Marcus’ descriptive language, and ability to explain this subject matter to a wide audience.

Scar as a villain? It seems Disney wasn’t far off when naming the bad guy in The Lion King.
It has been said that time heals all wounds, but what if the healing never stops? What if after wound healing and scar formation, the body cannot stop making more scar tissue, replacing healthy tissue with scar tissue until organ failure is inevitable? A terrifying thought, yet a reality for millions worldwide. It seems counterintuitive that a mechanism designed to save us from injury could be detrimental, however this is the definition of fibrosis, the cause of a staggering 45% of mortality in the Western world, a bigger killer than cancer yet much less famous. Given recent relevance, pulmonary fibrosis or lung fibrosis has been shown to be one of the long-term consequences of COVID-19, making it timely to explore it in more detail.
To understand fibrosis, we must understand wound healing. Wound healing itself is a highly conserved process in mammals since we cannot regenerate after damage (unlike reptiles). Consequently, this is safeguarded tightly, with many fail safes to ensure it works when needed. After blood clotting, wound healing can be divided into three distinct phases: inflammation (cells react to injury), proliferation (cells start to multiply), and remodelling (cells start to reform into tissue). These processes require complex relationships between various cell types (Figure 1).
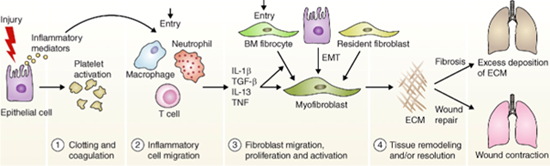
Figure 1: Mechanism of fibrosis formation
Wound healing itself is designed to fix an issue quickly rather than perfectly, which is why scar tissue has less of the original functionality. Out of control wound healing in which cells continue to multiply and reform can be fatal and is called fibrosis. This article will shed some light on the success (and lack thereof) in drug discovery in fibrosis.
Fibrosis can affect any organ or system in the human body, being particularly damaging in vital organs, which makes fibrosis one of the leading causes of death (Figure 2).

Figure 2: Many of the leading causes of death have a fibrotic component (e.g. ischaemic heart disease, chronic obstructive pulmonary disease, kidney disease)
Pulmonary fibrosis has a higher occurrence than small cell lung cancer with 13 to 20 patients per 100,000 worldwide and novel treatments have been suggested to generate $2 billion per year. These numbers are already impressive but do not factor in the impact of COVID-19, yet. Whilst any infection can lead to damage to the lungs, the mechanism of how COVID-19 causes lung fibrosis is still unclear. Hypotheses range from bacterial co-infections to cytokine storms (uncontrolled and excessive inflammation). A recent analysis has found that a staggering 45% of COVID-19 survivors appear to have developed lung fibrosis. Whilst COVID-19 severity is a major factor for developing lung fibrosis after infection, it is worth bearing in mind that since the COVID-19 outbreak around 293 million people have been infected and 256 million have recovered. This would potentially yield up to 115 million patients with pulmonary fibrosis, none of whom can receive effective treatment.
Drug discovery challenges
Due to the lack of safe and effective drugs, treatment for any fibrotic disease is mostly palliative (pain relief without curing), highlighting the clinical need for medical treatments. Currently there are only two drugs that have FDA-approval for the treatment of fibrosis in the lung: pirfenidone and nintedanib. Pirfenidone has anti-inflammatory and anti-fibrotic action and was first described in patents in the 1970s, yet its precise mechanism remains incompletely understood. However, a large amount of its anti-fibrotic action has been attributed to its inhibition of production and activity of a particular growth factor called Transforming growth factor beta (TGF-β).
Nintedanib targets three mechanisms (signalling pathways) which also have important roles in fibrosis. It was identified in large drug screening tests as a very specific inhibitor of certain enzymes that can switch cell functions on and off. But both drugs only yield modest results. Whilst improving one-year survival they are still only stalling the disease until an organ transplant becomes available.
So why is it, that despite the clear clinical need, there are only two drugs?
There are many challenges around drug discovery in the field of fibrosis (Figure 2). These challenges range from poor translation of lab results to the real world, the slow worsening of the disease and patient-to-patient differences in the disease, badly designed clinical trials, and too many candidate drugs to test in too little patients.

Figure 3: Challenges and solutions in fibrosis drug discovery
Additionally, our knowledge of how fibrosis works is still incomplete. Fibrosis is very complex, which highlights that single drugs targeting single pathways are unlikely to succeed, because of compensatory mechanisms taking over when only one pathway is targeted. While targeting the pathways that drive late progression of the disease has been suggested, these are also the pathways that drive tissue maintenance, increasing the risk of side effects.
Another key factor in clinical trial design is whether a drug aims to prevent the formation of fibrosis or whether it will treat already established tissue fibrosis (Figure 3).
Drugs, like nintedanib and pirfenidone, that can delay progression will improve the quality of life and stall the need for an organ transplant in patients with early-stage fibrosis. Patients with advanced fibrosis will require drugs that restore normal tissue architecture and reverse the established fibrosis. Lastly, there are patients in a pre-fibrotic stage wherein drugs with relatively little adverse effects are administered long term to prevent the formation of fibrosis. In these cases, the detection of at-risk patients will be key.
Currently, only one patient type has medical treatments available.

Figure 3: The three types of patients and their treatment needs
There are a number of clinical trials ongoing to find new drugs to treat or prevent fibrosis but many of these had to be terminated due to the side effects, another big issue for drug development.
Conclusion
Despite decades of research, the story of drug discovery in fibrosis cannot be classed a success, yet. With considerable economic pressure and strain on the healthcare systems expected (in part due to COVID-19), it is vital that we find novel treatments for fibrosis. The problems with drug discovery and the complexity of fibrotic disorders have been recognised and maybe it’s time to take a cue from other complex diseases. This could lead to a strategic change such as clinical trials testing drug combinations. Doing so led to major advances in cancer and HIV treatments and might help, along with other more innovative approaches, to finally beat the villainous scar.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.