As pharmacologists, we know that studies using research animals have been instrumental in the discovery and development of new medicines and in our understanding of the physiological mechanisms underlying health and disease. The recent approvals of several new vaccines for COVID-19 would not have been possible without using research animals in preliminary studies of their potential efficacy and safety. Despite promising scientific advances in non-animal alternatives, the use of research animals will continue for the foreseeable future. It is therefore important that those studying pharmacology and other biomedical degrees, and researchers in their early careers, understand how and why research animals are used. They should be able to verify the scientific validity of such studies and recognise the scientific and ethical reasons for conducting these to the highest welfare standards.
To support this aim, the British Pharmacological Society recently led a team of academic, welfare and industrial experts to develop a curriculum for the use of research animals. The ‘curriculum’ is a series of intended learning outcomes, which the Society hopes can be incorporated into teaching modules as widely as possible. The curriculum has two parts:
-
The core curriculum – aimed at undergraduate and taught Masters programmes or students that use data or literature derived from research animals. This can be delivered in the classroom setting and does not require any specialist laboratories.
-
The experiential curriculum – an additional component for those students who aspire to use research animals in their projects and careers. This involves some experiential training, either hands on and/or through the use of simulations.
Both curricula have been endorsed by the Physiological Society and a number of other learned Societies, welfare organisations, industry and universities.
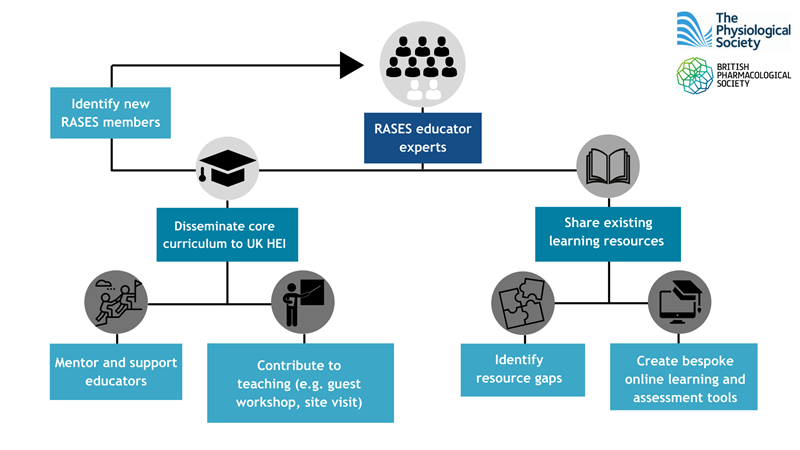
Although the curriculum is relevant to a large number of students studying biological sciences, there are only a few universities currently providing in-depth education in this important area. Lecturers in many institutions may only have limited personal experience of using research animals and may feel ill-equipped to deliver all parts of the curriculum. The Research Animal Sciences Education Scheme (RASES) is a joint project sponsored by the British Pharmacological Society and the Physiological Society to address this issue. The aim of RASES is to support implementation of the curriculum across a wide range of universities, including those with limited background in the area. The long-term goal is that education in the use of research animals is delivered to a more consistently high standard across universities throughout the UK.
Dissemination and support: RASES has recruited a number of academics (Table 1) from Higher Education Institutions who already have established expertise in delivering the key learning outcomes from the ‘curriculum for the use of research animals’ to their students. These ‘education experts’ will be available to collaborate with any university educators who have an interest in better embedding this topic into their programmes, or to improve their approach in teaching it. This support is free and can be tailored to the needs of the educator seeking it.
.png.aspx?width=800&height=450)
| Dr Susan Cochran |
University of Manchester |
| Dr Christine Edmead |
University of Bath |
| Dr Hui-Rong Jiang |
University of Strathclyde |
| Professor Simon Kennedy |
University of Glasgow |
| Dr Aileen King |
Kings College London |
| Dr Dave Lewis |
University of Leeds |
| Professor Emma Robinson |
University of Bristol |
Table 1. RASES Educator Experts
Examples of the type of support that could be offered include:
-
training sessions for educators e.g. innovative and effective ways to teach ethics
-
provision of existing materials e.g. an outline of a tried and tested workshop
-
opportunities for collaborative teaching e.g. guest lectures or tours of animal facilities and lab visits
-
signposting and sharing of research expertise e.g. provision of a case study for the use of research animals in a particular disease area
-
recommended online resources available to support teaching. A start has already been made and these will be continuously updated.
Evaluation
It is important that we measure the value and impact of the RASES support. To facilitate this, a survey has been developed to assess students’ understanding of key learning outcome statements within the curricula. The intention is that the survey will be conducted before and after completion of the university course to ascertain the extent of improvement in overall understanding. We also plan to develop a survey for educators, to assess how effective the RASES has been to increase their confidence in providing education in this area. This will also permit the collection of data on the various ways in which the learning outcomes have been implemented within different organisations.
Preliminary discussions have been held with 19 universities and the majority have expressed interest in accessing RASES support. Some institutions may develop new courses from scratch and may wish to use the ‘curriculum for the use of research animals’ as a framework for content and assessment creation. However, it is likely that the majority will be adding components to pre-existing courses. We know that timetables are full and students and staff have limited time available. Therefore, part of the role of the RASES educational experts is to identify ways to embed as many learning outcomes as relevant to the course, without it being too onerous or time consuming, whilst also being personalised to the needs for the educators.
Much of 2021 will be spent engaging with the initial group of institutions and assessing the benefits to the educators and students. If this pilot is successful, the RASES scheme will be widened to academics in other institutions. The scheme is currently fully funded by the British Pharmacological Society and the Physiological Society and is available at no cost to educators and institutions who wish to develop or discuss their teaching related to the use of research animals. If you are interested in RASES and would like to be involved as an educator expert or a recipient of support, please contact education@bps.ac.uk.
Examples of RASES support
Example 1
Dr Hui-Rong Jiang has provided Dr David Walsh at Caledonian University of Glasgow with lecture slides for animal research ethics and Ms Linda Horan has provided tours of the animal facility.
Student comments:
“The lectures were interesting and different and the trip to the animal facility was fascinating. Very important that students get to do this as it may help with our future career choices or research directions. Thank you.”
“The visit to the BPU really helped me to understand what goes on inside an animal facility, it greatly enhanced the lecture material from Dr Walsh and Dr Horan. Very enjoyable and informative”
Example 2
Professor Emma Robinson has been working with Dr Joan Jarman at Kingston University to set up an online behavioural pharmacology practical to run in their pharmacology unit. They have also discussed Professor Robinson giving a guest lecture, developing more practical sessions and creating materials on research integrity.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.