The number of Food and Drug Administration (FDA) approvals per $1bn spent on drug development halved every 9 years between 1950 and 2010 (Scannell et al., 2012). ‘Eroom’s Law’ was the term chosen to describe this notable attrition within the pharmaceutical industry. However, the demand for novel therapeutics is ever-increasing, with the pressure for new antibiotics rising amidst the global crisis of antimicrobial resistance (Murray et al., 2022). The developmental process of new drugs takes an average of 10-15 years and $2bn, and with only 10% of these reaching FDA approval. A more efficient approach to Research and Development (R&D) is required (Hinkson et al., 2020). This has led to the emergence of drug repurposing (DR) as a focus point of drug development (Rudrapal et al., 2020) (Zhan et al., 2022).
Also known as drug repositioning or reprofiling, DR refers to the identification of novel indications for existing drug compounds. It is not a novel concept, with many of the industry’s greatest successes originating from repositioning efforts. Aspirin, now the most commonly used drug worldwide, was first repositioned in the 1980s as an antiplatelet agent and has since gained increased recognition for its anticancer effects (Desborough & Keeling, 2017). In addition, sildenafil was first developed in the 1980s for use as an antihypertensive and an antianginal (Dhir et al., 2020). The subsequent discovery of its marked side-effects, however, led to its repositioning as a revolutionary treatment for erectile dysfunction in 1998 (Goldstein et al., 2019).
More recent DR endeavours include the numerous trials conducted during the COVID-19 pandemic such as DisCoVeRy and SOLIDARITY which sought to investigate several pre-approved drugs for the treatment of hospitalised patients. This led to antiviral medications remdesivir and molnupiravir receiving approval for emergency use in the treatment of SARS-CoV-2 (Hernán & Del Amo, 2023). Further success was found in the reprofiling of immunomodulators such as JAK inhibitor baricitinib and IL-6 inhibitor tocilizumab, both of which had been initially approved for the treatment of rheumatoid arthritis (Marconi et al., 2021) (Rosas et al., 2021). With both medical professionals and the pharmaceutical industry under pressure to produce rapid and effective treatments for the critically ill, DR served as an attractive and efficient strategy. This was greatly aided by the adoption of a data-driven approach, using machine learning and computational mapping to identify successful compounds from heterogenous data (Duarte et al., 2020) (Ge et al., 2020). The introduction of artificial intelligence (AI) into the DR process during the pandemic was largely beneficial and likely to become an integral tool in the prediction of potential candidates for repositioning (Zhao et al., 2022).
Despite its high-profile success stories, the benefits of DR have come under scrutiny. The probability of identifying a candidate for reprofiling is often questioned. While it may be argued that many DR successes resulted from serendipitous discoveries, the application of in silico approaches combined with the screening of compound libraries has streamlined the process of candidate identification (Cha et al., 2018) (March-Vila et al., 2017). Moreover, once identified, the chances of DR success exceed novel drug development 3-fold with an estimated 30% approval rate reported for pre-existing drugs, the majority reaching approval within 3-12 years (Hernandez et al., 2017), compared to a mere 10% for New Molecular Entities (NMEs). This increased turnover rate is vital in allowing for pharmaceutical innovation to keep up with increasing demand.
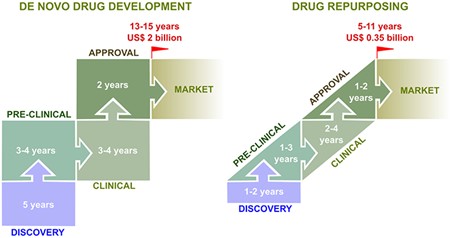
Fig. 1. The development pipeline of de novo and repurposed drugs from concept to market.
DR also serves as an opportunity for investors to recoup some of the loss on projects claimed by the so-called ‘valley of death’. An estimated 40-60% of failures occur following phase III trials due to lack of efficacy (Sun et al., 2022). With the mode of action and toxicity profiles of these shelved drugs already understood, the main objective in DR is finding a suitable indication or disease, rather than starting from scratch. This saves both time and money, costing on average $300m (Pushpakom et al., 2019) due to the reduced need for regulatory approval and may allow for the generation of capital for future endeavours, with DR accounting for an estimated 25% of pharmaceutical revenue in recent years (Rudrapal et al., 2020) .
Intellectual property (IP) concerns are frequently cited as limitations to DR profitability. New Memorandum of Understanding (MOU) patents may be obtained for existing drugs if the indication is proven to, in fact, be new (Pushpakom et al., 2019). However, due to ‘off-label’ prescribing being common practice amongst physicians, many alternative indications for existing drugs are already within the public domain. Difficulty in obtaining secondary patents may limit the financial gain from DR projects and serve as a deterrent for investors. However, with strategic planning, IP may be exploited as an advantage. In the case of rare diseases, repurposed drugs may benefit from 10 years of EU market exclusivity under the Orphan Drug Act, independent of their current patent status (Pushpakom et al., 2019). This holds the potential to enhance the financial return from old compounds, particularly those reaching the end of their 20-year patent protection. Moreover, secondary cosmetic indications provide the option of licensing to third parties, allowing for the generation of new profit channels. Such was the case with minoxidil, initially developed for the treatment of angina but repurposed for use in male pattern baldness.
A decrease in R&D attrition has been observed in recent years (Pammolli et al., 2020). This cannot be fully attributed to DR, with the use of surrogate trial endpoints and genetic validation of targets also impacting on R&D productivity (Nelson et al., 2015) (Schilsky et al., 2012). However, with an estimated 30% of FDA approvals originating from DR, its vital role in drug development is clear (Rudrapal et al., 2020). To rely on de novo discovery alone would be a Sisyphean task. Hence, the term ‘saviour’ seems apt to describe the role of DR in drug development.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.