Rosemary Gaw, University of Strathclyde, was a runner up from our 2021 annual writing competition for early career pharmacologists. For each writing competition, we look for exceptionally written and well-communicated science on any pharmacology-related topic. Each of our entries was scored independently by a panel of judges*.
What if there were a way to fix the root cause of disease? What if we could look at the individual patient and say, “this gene is causing your illness, here is a medicine that will make you better”? Precision medicine is emerging as a way to optimise treatment based on individual patient characteristics. In the UK, around 1 in 2 people develop cancer in their lifetime. Cancer is at the forefront of precision medicine, with many cancerous tumours now classified according to the presence or absence of specific genetic mutations. This means that treatments can target the root cause – in this case the mechanisms underlying cancer cell growth and survival.
Sir George Thomas Beatson (1848-1933) was a Glasgow-based surgeon credited with the discovery that ovarian function can drive some breast cancers. He demonstrated that removal of the ovaries resulted in marked improvement in three patients with advanced breast cancer. This was a revolutionary idea during an era when cancer was widely thought to be parasitic in nature and the term ‘hormone’ did not yet exist. His discovery led to removal of the ovaries becoming standard practice for many years. However, there was no way to predict whether the patient would benefit or not.

Figure 1. Sir George Thomas Beatson, 1848-1933.
Following on from Sir Beatson’s work, there were two key developments in the 1970s. A test to measure oestrogen receptor levels in breast cancer specimens predicted whether patients would benefit from reduced ovarian oestrogen production for the first time. Synthesis of compounds to target oestrogen receptor binding led to the production of Tamoxifen, a highly effective therapy which continues to benefit millions of women worldwide today.
A more recent development in precision medicine is in the treatment of leukaemia - a group of blood cancers originating in the bone marrow. Consider the example of 40 patients with acute lymphoblastic leukaemia, which affects the ‘lymphocyte’ blood cells. Before the advent of precision medicine, all 40 of these patients would have received standardised chemotherapy. In other words, patients with the same type of cancer at the same stage would receive the same treatment.
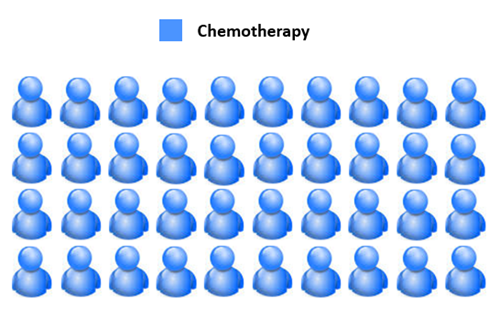
Figure 2. Before precision medicine, 40/40 acute lymphoblastic leukaemia patients would receive the same standardised chemotherapy.
Precision medicine identifies a specific faulty gene or molecule (known as a biomarker) by which the disease process occurs. Once identified, therapeutic drugs can be designed to specifically target this biomarker. These are known as targeted therapies and importantly, do not act on all cells but only those with the specific molecular target. It may be tempting to assume that for this reason, targeted therapies are unlikely to cause side effects. However, this is not the case, and they can be associated with skin reactions, inflammation, hair loss, and other side effects.
Going back to the example of acute lymphoblastic leukaemia, the Philadelphia chromosome is a specific genetic abnormality found in leukaemia cells in 20-30% of patients resulting from a translocation involving chromosome 9 and 22. This translocation produces a mutant gene called BCR-ABL1 which is always ‘switched on’ and produces a tyrosine kinase protein. This aberrant protein activity stimulates uncontrolled cancer cell division and growth. A targeted therapy called Imatinib blocks this abnormal tyrosine kinase, preventing further cancer cell division and growth. However, it will only act on cancer cells expressing BCR-ABL1. Thus, out of a sample of 40 patients up to 12 (30%) would be expected to benefit from this drug. Imatinib has been highly successful and has increased childhood acute lymphoblastic leukaemia survival rates from around 30% to 70%.
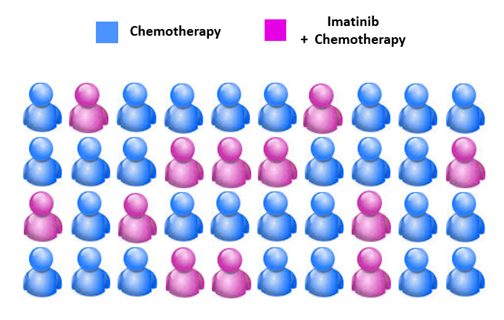
Figure 3. Up to 12/40 acute lymphoblastic leukaemia patients would be expected to have Philadelphia-positive leukaemia and thus, benefit from Imatinib.
Where is precision medicine going in the future?
Whilst current precision medicine therapies generally target subgroups of patients, new therapies at the individual level are starting to emerge. An example of this is CAR T-cell therapy for certain types of blood and lymphatic system cancers, where patients’ own T-cells are genetically engineered to enhance their natural ability to detect and kill cancer cells.
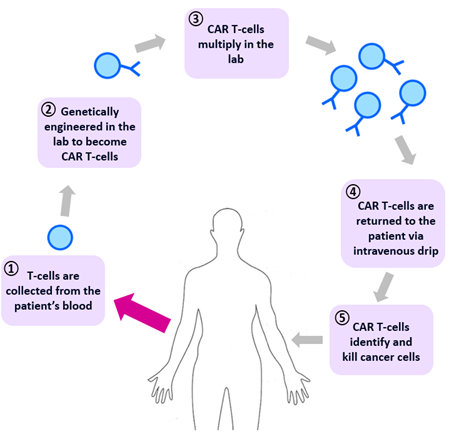
Figure 4. CAR T-cell therapy. T-lymphocytes are collected from the patient’s blood, genetically engineered to express the chimeric antigen receptor (CAR) and multiplied in the lab. The CAR-T cells are then returned to the patient where they identify and kill cancer cells.
Whilst cancer remains at the forefront of precision medicine, it is important to note that significant advances continue to be made in other conditions. For example, IgE is an inflammatory chemical produced when a person has an allergic reaction. Some patients with severe asthma have an allergic subtype characterised by high levels of IgE, causing inflammation in the lungs. Omalizumab is a monoclonal antibody which blocks IgE production, improving asthma symptoms and reducing the risk of a life-threatening asthma attack. However, the NHS have strict eligibility criteria including serum IgE levels of 30-1500 IU/mL as non-allergic asthma subtypes are unlikely to respond to Omalizumab.
Is precision medicine really a ‘magic bullet’?
The Oxford English Dictionary defines a magic bullet as ‘a medical treatment that works very quickly and effectively against a particular illness’. Precision medicine is certainly effective, as the remission rates of up to 90% following CAR T-cell therapy show. However, patient access remains a concern due to the high costs. For example, Kadcyla - a life-extending drug for advanced HER2-positive breast cancer - was withdrawn from use within NHS England in 2017, citing an unfavourable cost-benefit analysis of £90,000 per patient versus an average six-month extension to life. This caused significant distress to patients, especially as Kadcyla was simultaneously made available within NHS Scotland,resulting in a ‘postcode lottery’ situation.
In conclusion, precision medicine has a great deal of potential. However, it won’t be a magic bullet until patient access is improved through increased funding for rare diseases, reduced drug development costs, and increased collaboration between pharmaceutical companies and healthcare funding bodies.
*This year’s judging panel was made up of: Craig Daly (Pharmacology Matters editor), Hannah Roughley (Pharmacology Matters editor), James Boncan (Pharmacology Matters editor), Josh Dignam (Pharmacology Matters editor), Lauren Taylor (Pharmacology Matters editor), Sorrel Bunting (then Engagement Manager at the Society), Taichi Ochi (Pharmacology Matters editor), and Sarah Bailey (Vice President Engagement at the Society).
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.