On 4 October 2021, the Nobel Committee announced that Professors David Julius and Ardem Patapoutian had won the 2021 Nobel Prize for Physiology and Medicine for their ground-breaking discoveries of the TRPV1, TRPM8 and Piezo ion channels involved in pain, heat, and touch sensation.
A year of lockdowns and social distancing has resulted in many missed opportunities for interaction, and it is exciting to see people reconnecting. As we start to meet again in-person, the importance of our interaction with our surroundings and other people is clearer than ever. It is fitting therefore, that this year’s Nobel Prize in Physiology and Medicine has been awarded for research into human sensation, particularly into how we perceive pain, heat, and touch.

.jpg.aspx?width=300&height=200)
Professor David Julius (left) and Professor Ardem Patapoutian(right) are this year’s recipients of the Nobel Prize in Physiology and Medicine. Photographs: Noah Berger and Scripps Research Collection.
While many of us take our senses for granted, David Julius, a Professor in the Department of Physiology at the University of California San Francisco, and Ardem Patapoutian, a Professor in the Department of Neuroscience at Scripps Research Institute in California, have spent years trying to better understand how we sense pain, temperature, and touch. Our senses are so integral to our understanding of the world and our everyday lives, yet until recently, very little was understood about the mechanisms underlying these senses.
A burning question
Although all our senses are important for our perception of our surroundings, nociception (the perception of noxious stimuli) is vital for ensuring that we can sense and avoid harm. If exposure to noxious stimuli (such as harmful chemicals or high heat) reaches a certain excitation threshold, an action potential is fired along nociceptive neurons, such as the Aδ and C fibres. This signal is then transmitted to the dorsal horn of the spinal cord before reaching the brain, resulting in the release of neurotransmitters and inducing the sensation of pain. This response is designed to alert us of danger and ‘teach’ us to avoid potential tissue damage in the future.
To better understand how we feel and sense pain and temperature, Professor Julius began investigating capsaicin - the chemical found in chilli peppers that gives them their pungency. Whilst it was already established that capsaicin could excite nociceptors to cause the release of neurotransmitters (e.g. substance P and calcitonin gene-related peptide), the receptor responsible for this action was unknown. To identify the ‘capsaicin receptor’, Professor Julius and his colleagues began by using expression cloning assays. Firstly, a cDNA library was generated from nociceptive neurons in the sensory ganglia. From this library, cDNA clones were transfected into non-neuronal cells that did not normally respond to capsaicin. Professor Julius and co-workers then exposed the transfected cells to capsaicin and measured the changes in intracellular free calcium release. After several rounds of selection, in 1997, Professor Julius and colleagues successfully isolated the clone they were looking for and found that it encoded a novel ion channel protein, later named TRPV1 (transient receptor potential cation channel subfamily V member 1). Further experiments on the TRPV1 channel revealed that it is largely expressed in C fibres and does not just react to capsaicin but can also be activated by noxious heat of above 42⁰C, inflammatory mediators, and high proton concentrations of pH 5.5 and below.
While acute pain serves as a guarding mechanism against further injury, chronic pain (for example neuropathic pain) can often be debilitating and difficult to treat effectively. The discovery of the TRPV1 channel therefore sparked interest in targeting the receptor to treat chronic pain. Interestingly, research has shown that despite its action to induce pain upon initial exposure, repeated or prolonged exposure to capsaicin desensitises nerve fibres expressing TRPV1, leading to analgesia and reduced sensitivity to noxious stimuli. While systemic administration of capsaicin may cause irritation and increases in body temperature, capsaicin creams and dermal patches are available in the clinic for use in neuropathic and musculoskeletal pain. Research is also exploring TRPV1 antagonists as analgesics, however evidence from clinical trials has shown side effects such as hyperthermia, for example in Amgen’s Phase 1b clinical trials of their AMG517 molecule. Other antagonists, such as the Merck/Neurogen MK-2295 molecule, have also been associated with a decrease in the ability to sense noxious heat, resulting in increased occurrences of scalding injuries among clinical trial participants. As such, it is possible that future TRPV1 antagonists may need to be administered as topical formulations to avoid such systemic side effects.
Further discoveries are made
The identification of the capsaicin receptor paved the way for the discovery of other temperature-sensing ion channels in the TRP channel family. By turning back to nature for inspiration, Professors Julius and Patapoutian (independently of each other) would both go on to use menthol to identify the cold and menthol-sensitive receptor, TRPM8 (transient receptor potential melastatin 8). Upon discovery, both found that TRPM8 is responsible for the cold sensation felt upon both noxious and innocuous stimuli, with the channel having an activation threshold of <26⁰C. Research into targeting the TRPM8 channel has suggested that antagonists may be useful in relieving cold-induced pain, chemotherapy-induced cold allodynia, and migraines. However, varying results have been seen with antagonists to date, with molecules such as Pfizer’s PF-05105679 candidate molecule resulting in adverse hot sensations.
Additional work by Professor Julius also identified the TRPA1 channel (transient receptor potential ankyrin 1), a cold sensor which is responsible for perception of pain in response to pungent reagents such as mustard oil and cinnamaldehyde. Interestingly, it has been shown that a large proportion of TRPV1-expressing C fibres also express TRPA1, which have been shown to cross-react in their nociceptive responses and are important for pain sensation. Similarly to TRPV1, the TRPA1 channel has also been associated with neuropathic pain and inflammation, leading to interest in targeting TRPA1 in pain conditions. However, only one TRPA1 antagonist has made it into phase II clinical trials thus far – Glenmark’s GRC17536 molecule, which showed some analgesic effect in diabetic polyneuropathy, but did not advance to phase III trials due to bioavailability issues.
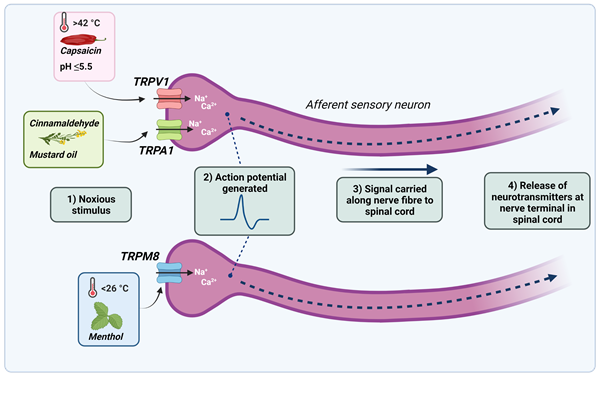
Figure 1. Noxious stimuli cause the TRPV1, TRPA1 and TRPM8 ion channels present on sensory neurons to open, allowing an influx of calcium and sodium ions. If the threshold for an action potential is reached, an action potential is generated and propagated along the length of the nerve. Neurotransmitters are then released at the nerve terminal in the spinal dorsal horn, where the signal is transmitted to the brain and the sensation of pain is felt.
Feeling the pressure
While the pathways of pain and temperature sensation were beginning to unravel, the mechanisms in which cells interpret mechanical stimuli (mechanotransduction) such as pressure and touch, remained elusive. Aside from being essential to our understanding and interpretation of our surroundings, mechanotransduction is also important in many aspects of our physiology, for example in our ability to hear, sense pain, and regulate blood pressure.
To better understand mechanotransduction, Professor Patapoutian and his colleagues began their work by utilising a pressure-sensitive cell line to perform microarray analyses of a list of candidate ion channels. They then knocked-down the expression of these candidates using siRNA (short-interfering RNA) to test whether each gene was necessary for mechanotransduction. They then poked cells with a micropipette to measure the generation of mechanically-activated currents. In 2010, after many experiments, Professor Patapoutian and his colleagues successfully found the gene that causes response to pressure and gave it the name ‘Piezo1’, derived from the Greek word for pressure ‘πίεση’ (pίesi). Professor Patapoutian then went on to discover a second Piezo channel, named Piezo2. Later research identified that the Piezo channels were not only involved in touch sensation, but also in proprioception (the sense of where your limbs are in space) and interoception (the sensing of internal organs, for example sensing bladder fullness).
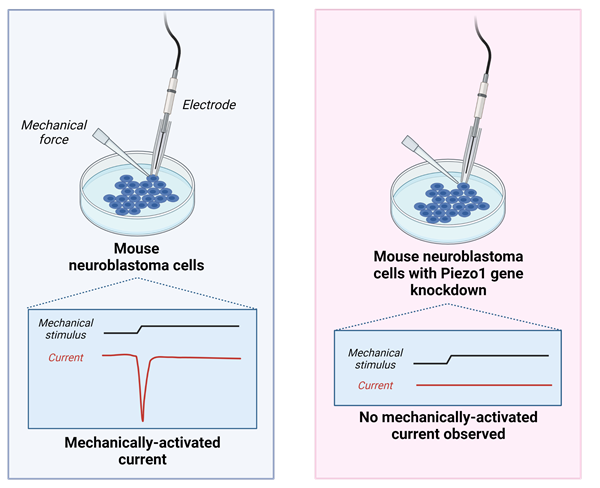
Figure 2. Patapoutian and colleagues observed that in mouse neuroblastoma cells, cells expressing the Piezo1 gene generated a mechanically-activated current when poked with a micropipette, while cells with Piezo1 gene silencing did not.
Since their discovery, the Piezo channels have become exciting targets in the treatment of chronic pain conditions as evidence has shown an association between Piezo2 and allodynia (experiencing pain from non-painful touch) in conditions such as fibromyalgia. Antagonists of Piezo2 may therefore serve as a useful mechanism of targeting chronic pain. However, similarly to the TRP channels, antagonists would need to be administered topically or locally to avoid systemic loss of touch sensation.
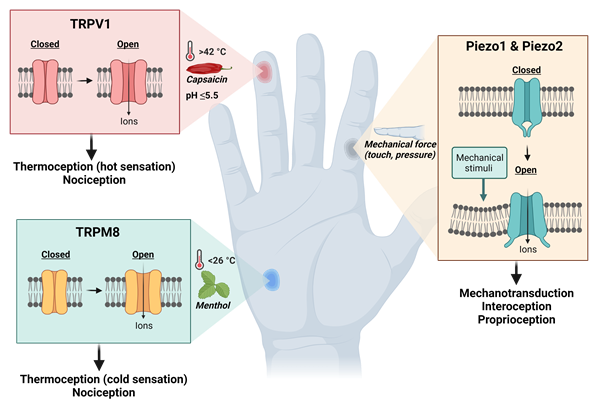
Figure 3. The TRPV1, TRPM8 and Piezo ion channels involved in the sensation of pain, thermal and mechanical stimuli. Images created with Biorender.com.
Professors Julius and Patapoutian’s discoveries have been essential in our understanding of pain, temperature, and touch, however, these discoveries have also revealed novel targets for the treatment of chronic pain conditions. Such conditions remain challenging for medicinal intervention, and many analgesics, such as opioids, also exhibit severe side effects due to their actions on the central nervous system. Therefore, while the discoveries of the TRP and Piezo channels have assisted in the general understanding of pain and touch, they also remain exciting as novel targets for analgesia.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.