There are approximately 60,000 miles (100,000 km) of blood vessels in our body. The larger vessels branch off into ever smaller segments until they reach the micro scale, where curious behaviours emerge. Micro-sized blood vessels often directly interact with organ-specific tissues to deliver vital supplies such as oxygen and nutrients and remove cellular waste – the ultimate operation in logistics and sanitation.
A lack of access to human tissues for research purposes has been a frustrating blockade for pharmacologists, vaccine researchers and basic scientists alike. At some point they each would have wondered: if I can’t access these tissues, then why don’t I create them? But the reality is that recreating the architecture and hierarchy of vessels is a significant challenge. There are multiple approaches that could be taken, however any potential industrial adoption of a new technology must consider whether the technology is accurate, scalable and robust enough to recreate features of healthy or diseased tissues.
Drawing inspiration from nature or from pre-existing technologies is the path of least resistance. Computers and smartphones have become more powerful over the course of our lifetimes, due to advances in computer processing. The same technology used to create micro-scale and nano-scale circuitry behind these processing advances has been hybridised with biomedical research to create micro-sized channels, inside which cells can be cultured and tissue culture medium can be perfused.
Using the exact geometry of blood vessels allows researchers to study their function and how specific cell types respond to a range of stimuli in controlled conditions.
The van der Meer group at the University of Twente used scans of healthy and diseased human coronary arteries to recreate thrombotic events inside 3D-printed microchips that resemble the conditions in a patient’s blood vessel.
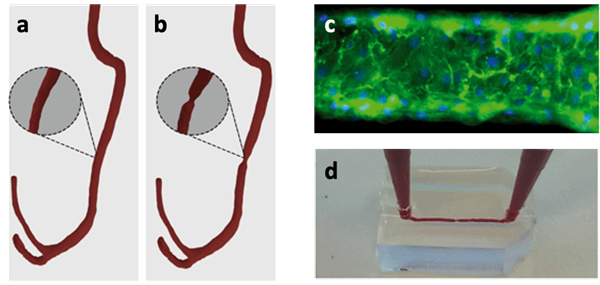
3D rendering of (a) healthy coronary arteries and (b) diseased coronary arteries based on computed tomography (CT) scans from individuals; (c) fluorescent images of endothelial cells (which typically line the innermost layer of blood vessels) inside the microfluidic device; (d) introduction of blood into a 3D-printed transparent microfluidic device to mimic the flow of blood. Images reproduced from Costa et al., 2017. Published by The Royal Society of Chemistry (RSC) on behalf of the Centre National de la Recherche Scientifique (CNRS) and the RSC.
High levels of vascularisation are the foundations on which organs can perform specialised functions. Blood vessels replenish oxygen, remove waste and transport signalling molecules to and from tissues. Also important is the interaction of different cell types and physiological characteristics such as fluid flow or mechanical stretching. In standard biomedical laboratory practice, monocultures of individual cell types are cultured in static environments that do not represent the environments they were isolated from, resulting in aberrant behaviour. The alternative has been to use animal research models, which, despite the ethical implications, have undoubtedly saved or enhanced countless lives.
Biological differences between animals and humans are an obstacle to pharmacological and toxicological safety studies of novel compounds. Drugs that pass stringent animal testing can cause severe damage, sometimes even death, to humans. Where animal testing is considered a necessary but unavoidable part of research, the theory is centuries old. Particularly in science, where progress can be measured in weeks and months, it seems strange that animal research was a feature of some of the earliest work on physiology by eminent scholars such as Aristotle or Galen (albeit today’s research has infinitely higher welfare standards and protocols).
Microfluidics offers scientists an approach to craft the biological tools to recreate human biology which can circumvent direct human testing. The Ingber research group at the Wyss Institute for Biologically Inspired Engineering pioneered the development of organs-on-chips: vascularised microfluidic devices that also contain organ-specific tissue to recapitulate key organ level functions. Their first model, a ‘breathing’ lung-on-a-chip, recreated the interface between the capillary and the alveolar air sac, successfully modelling the human response to invading pathogens.
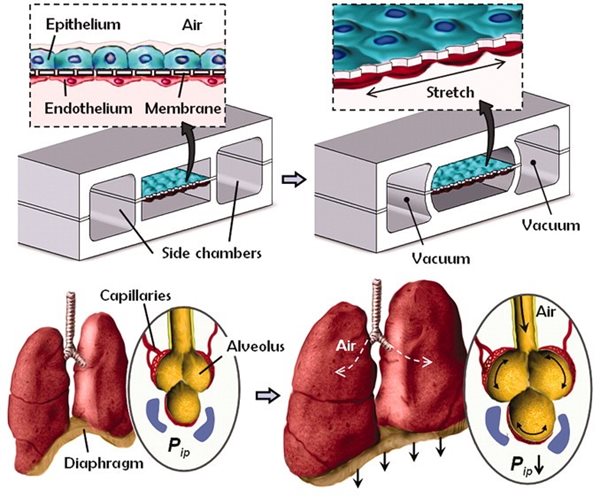
A “breathing” lung-on-a-chip. Cross-sectional schematic showing the layout of microchannels within the microfluidic device. Epithelial cells (representing the alveolar air sac) and endothelial cells (representing the capillary) are cultured either side of a stretchable porous membrane. Cells are cultured at an air-to-liquid interface, just as is seen in the body. Cyclic vacuum is applied to hollow side chambers which mechanically stretches the cell culture surface and mimics the motions of breathing. From Huh et al., 2010. Reprinted with permission from AAAS.
Readers may view, browse, and/or download material for temporary copying purposes only, provided these uses are for noncommercial personal purposes. Except as provided by law, this material may not be further reproduced, distributed, transmitted, modified, adapted, performed, displayed, published, or sold in whole or in part, without prior written permission from the publisher.
After demonstrating microfluidic replication of classical human lung responses the lung-on-a-chip was able to model pulmonary edema – where the air-to-liquid interface is compromised, leading fluid to enter the alveolus – in a physiologically relevant man-made environment, which had previously not been possible in a lab setting. Therapeutic intervention was then able to prevent the onset of pulmonary edema, kicking off the next biological gold rush to develop other models of organs on chips for toxicological screening. Hundreds of models now exist, targeting a variety of major organs and tissues ranging from gut-on-a-chip to pulmonary-artery-on-a-chip models.
The astounding predictive power of organs-on-chips was demonstrated in a study of a compound that had passed animal testing protocols and had entered into human clinical trials, leading to liver failure and death in a third of participants. Using a human liver-on-a-chip platform containing human liver cells, in parallel with a rat-on-a-chip platform containing rat liver cells, the study showed the compound caused human-specific toxicity while leaving the rat-chip unaffected, highlighting the dangers of placing too much confidence in results obtained from a species different to our own.
In organ-on-a-chip models that contain a vascular compartment that models blood transport, there is a vision to directly link two vascular compartments of two independent organ models which would simulate blood flow and transport of molecules between organ models. By extension, this system could serve as a fully human body-on-a-chip platform by fluidically linking multiple organ models to reveal entirely human responses to drugs, potentially eliminating the need for animal testing.
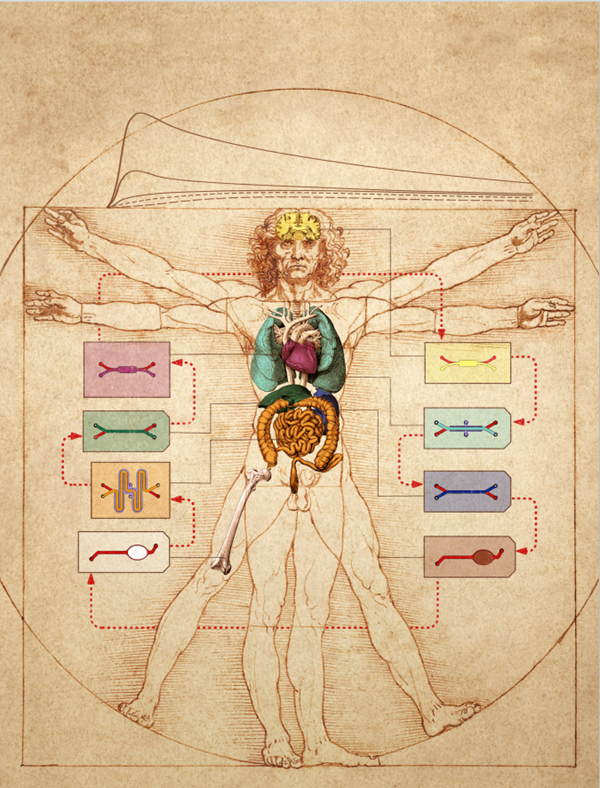
A body-on-a-chip platform overlaid onto Leonardo Da Vinci’s famous Vitruvian Man sketch. The interlinking of individual organs-on-chips through vascular channels (highlighted in red) to assemble a body-on-a-chip platform for pharmacological and toxicological purposes. Image credit: Wyss Institute at Harvard University.
The extraordinary predictive power of organs on chips is gaining momentum. It has long been known that the drug development model is broken. Billions of pounds are spent each year on the research and development of new drugs, however this investment all too often represents water circling a drain, captivating and full of promise but slowly disappearing out of sight.
Individually, these organs-on-chips have the potential to reduce the cost of developing and validating new therapeutics, however they are individual pieces of a biological puzzle. The holy grail for predictive drug screening, in which vascular networks are central, is an accessible and inexpensive body-on-a-chip platform. In this way, we could phase out animal testing. As Aristotle shrewdly postulated: the whole is greater than the sum of its parts.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.