The following article by Josh Dignam of the William Harvey Research Institute was selected by a panel of judges as the winner of Pharmacology Matters’ first writing prize for Early Career Researchers. Entrants were tasked with writing entertaining, original articles which could be appreciated by a lay audience. This article was selected for its exceptional development of ideas and flowing narrative.
Of Fangs and Pharmacology: A Deadly Cure for Heart Disease
To many people, snakes are the very antithesis of medicine. In fact the World Health Organization (WHO) estimates 2.7 million venomous snakebites occur annually, resulting in approximately 400,000 disabilities and 100,000 deaths. Yet, to pharmacologists, snakes are a potential treasure trove of new drugs. Snake venom contains a potent mixture of toxins designed to subdue prey and fend off predators. Over millions of years, each of these molecules has evolved to target a highly specific component of the circulatory or nervous system. Their deadly precision is the very reason venom toxins offer a strong foundation for developing new medicines. Indeed, when individual toxins are isolated and administered at an appropriate dose, they can be used safely and effectively as therapeutic agents. To date, three drugs have been successfully developed from snake venom, all for the treatment of heart disease.
Plantation workers bitten by the Brazilian pit viper (Bothrops jararaca) often faint due to a sudden drop in blood pressure. Its venom is so potent that indigenous tribes were said to apply it to their arrowheads. This local knowledge piqued the interest of Brazilian pharmacologist Mauricio Rocha e Silva and so he began to investigate how the bite of the pit viper induces vascular shock. In 1948, he discovered a substance that accumulates in plasma after addition of the venom. This polypeptide, which he called bradykinin, had the ability to relax blood vessels and reduce blood pressure. In the early 60s, his student Sérgio Henrique Ferreira isolated a substance (later identified as a peptide) from the venom that prevented the break-down of bradykinin and enhanced its blood pressure lowering potential. In a seminal paper published in the British Journal of Pharmacology, they termed this substance bradykinin potentiating factor (BPF).
In 1964, Ferreira travelled to London to join a group led by John Vane at the Royal College of Surgeons. At the time, Vane was actively investigating the cause of high blood pressure (hypertension). Contrary to the opinion of most clinical experts, he had a hunch that angiotensin-converting-enzyme (ACE) inhibition could treat hypertension. In the body, ACE cleaves an inactive precursor molecule (angiotensin I) into the hormone angiotensin II, which has a potent narrowing effect on blood vessels and consequently increases blood pressure. Using venom supplied by Ferreira, Vane’s colleague Y.S. Bakhle demonstrated that BPF blocks the activity of ACE. They correctly hypothesised that ACE was responsible for both the production of angiotensin II and break-down of bradykinin (Figure 1).
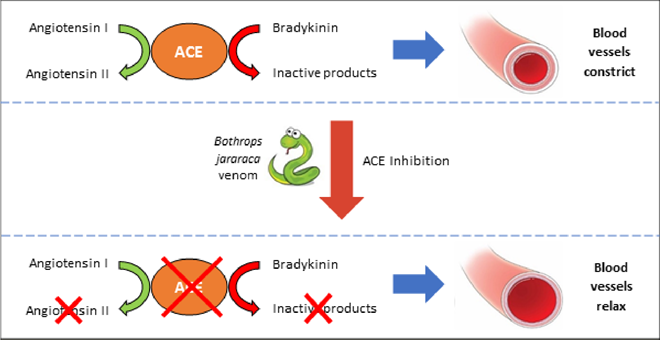
Figure 1 - An ACE Discovery: ACE inhibitors block the production of the hypertensive (i.e. blood pressure increasing) hormone angiotensin II and prevent the break-down of the hypotensive (i.e. blood presuure lowering) peptide bradkinin. The overall effect is widening of the blood vessels and lowering of blooding pressure. This makes it easier for the heart to pump blood and can improve the function of a failing heart.
In 1967, Vane presented his ideas to pharmaceutical company Squibb (now Bristol‐Myers Squibb) and encouraged them to start searching for inhibitors of ACE. In response, a team in New Jersey led by David Cushman and Miguel Ondetti began to isolate and purify peptides from the venom. Of note was teprotide, which became the first ACE inhibitor trialled in patients with hypertension. Despite promising results, development was halted. Like most peptides, teprotide is only effective when given as an injection, and therefore unsuitable as a routine therapy.
Following the unsuccessful screening of a further 2000 compounds, the project was on the brink of cancellation. The lucky break came in 1974. A paper was published describing benzylsuccinic acid, a potent new inhibitor of carboxypeptidase A, an enzyme with a structure remarkably like that of ACE. In discussing the paper, Cushman and Ondetti made several conceptual breakthroughs that facilitated systematic and targeted alterations to the structure of terprotide. One and a half years and 60 compounds later, they struck gold: a small molecule ACE inhibitor that could be given by mouth - captopril. In 1981, it became the first venom-inspired drug to gain approval from the Food and Drug Administration. Today, ACE inhibitors like captopril remain a first-line therapy for hypertension and heart failure. In fact, of the 70.1 million anti-hypertensives prescribed annually in the UK, approximately 32% are ACE inhibitors.
The development of captopril not only advanced the treatment of heart disease, but also demonstrated that venom toxins are useful templates for drug discovery (Figure 2). In response, more and more scientists began to turn to snakes in the hunt for new medicines. This ultimately led to the development of two more drugs now used in the management of heart disease.
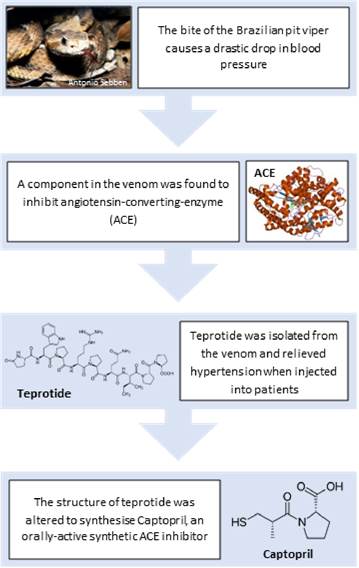
Figure 2 – Developing Drugs with Bite: To adapt a venom into a new drug, the active component must first be identified. Once the mode of action and molecular structure of this component have been determined, the structure can be modified as necessary to optimise the properties of the drug candidate.
In the 90s, a molecule known as barbourin was identified in the venom of the dusky pygmy rattlesnake (Sistrurus miliarius barbouri). Barbourin blocks a protein complex on the surface of platelets, preventing them sticking together and forming a blood clot (Figure 3). Using the structure of barbourin, a peptide called eptifibatide was developed. It is used to prevent clot formation in people with severe angina or undergoing a procedure to re-open a blocked artery. A similar toxin (echistatin) from the saw-scaled viper (Echis carinatus) is the basis of the drug tirofiban.
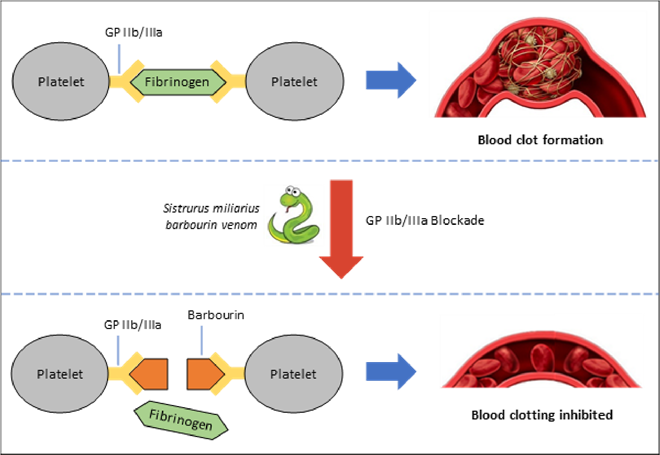
Figure 3 - Barbourin: Poison to Potion: To form a blood clot, fibrinogen (a factor in blood) binds to GP IIb/IIIa on platelets and links them together, forming a net which traps red blood cells, Barbourin blocks GP IIb/IIa, preventing clot formation. Clotting is essential for wound healing but when excessive can block blood vessels, causing a heart attack.
Over the past decade, the advent of more sophisticated and affordable technologies has made studying the structure and function of the bioactive molecules in venom easier than ever. Thus, whilst only three snake venom-inspired drugs have been approved to date, there are many candidates in the pipeline. Snake venom has proven a bountiful source of natriuretic peptides (NPs), possessing greater diversity, stability and potency than their mammalian counterparts. NPs are important regulators of cardiovascular function, and consequently are under investigation as a treatment for heart failure.
The first venom NP discovered was Dendroaspis natriuretic peptide (DNP), from the eastern green mamba (Dendroaspis angusticeps; Figure 4). DNP, which lowers blood pressure, was fused with another NP from human blood vessels, C-type natriuretic peptide (CNP), which protects and promotes repair in the heart. The resultant molecule, Cenderitide (CD-NP), exhibits the benefits of both NPs and has shown promising results in clinical trials of heart failure patients.

Figure 4 - Eastern Green Mamba (Dendroaspis angisticeps): These snakes inhabit coastal regions of South East Africa. They grow to an average of 6-7 feet and possess a bite that can kill within 30 minutes.
Venom represents one of the great paradoxes of the natural world. Fundamentally it’s lethal, yet the very properties that make it so can be exploited for the preservation of life. Since the introduction of captopril, snakes have arguably saved more human lives than they have taken. This has ignited interest in other venomous creatures, from lizards to leeches and snails to spiders. As such, the number of venom-derived drugs is only set to rise, with candidates in development for heart disease, diabetes and cancer. Of course, pharmacologists can only study these venoms if the species are there to find. The global increase in urbanisation is threatening many venomous species. If they go into the dark abyss of extinction, they’ll take their medicines with them.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.