Anitha Nair, a PhD student at William Harvey Research Institute, was selected by a panel of judges as the winner of the second annual Pharmacology Matters Writing Competition for Early Career Researchers. Her article was selected for being an informative and well-written exploration of an interesting, timely topic.
.jpg.aspx)
Many of us view aging with a sense of impending doom. The recent coronavirus outbreak is an example of how vulnerable we become as we age, as it was older people who were most affected. Aging is defined by a progressive decline in cognitive, psychosocial, motor-neuron and immune functions. Most people predict their old age to be a slow, frail and socially restricted existence. Fortunately, this need not be the case. Recent advances in aging research suggest that old age can be just another phase of life, rather than doom and gloom.
Nevertheless, the million-dollar question is, can you stop aging? The simple answer is no; aging is inevitable as it is , a complex mix of degenerative conditions with an unknown biological mechanism. But it can be delayed by transiently blocking the triggers of aging, using pharmacological approaches. Thus, the current research focus is primarily on improving quality of life by prolonging the ‘healthspan’, or active years of an individual.
To this effect, the World Health Organization is advocating a Decade of Healthy Ageing (2020–2030). It is bringing together governments, academia, healthcare and other organisations for 10 years of collaborative action to help people age without discomfort and with better quality of life. The aim is to treat or prevent age-related disorders (ARDs) like osteoarthritis, memory loss, cancer and respiratory-tract infections. However, the key is to identify all biological pathways of aging at the cellular and molecular level.
Aging, at the cellular level, is characterized by cell senescence, caused by DNA damage, loss of cell division and regeneration. These senescent or ‘zombie’ cells linger in the tissue, secreting harmful inflammatory factors that turn healthy neighbouring cells into more senescent cells. This leads to widespread tissue damage. Hence, the older a person gets, the less functional their body become, which significantly compromises their quality of life.
Dawn of senotherapeutics
Senotherapeutics are anti-aging drugs that target the triggers of cellular senescence, such as DNA damage, oxidative stress and metabolic stress, thereby alleviating ARDs. Several senotherapeutics are being studied worldwide, at different stages of development. This article will focus on the few that has been successfully tested in humans.
Dasatinib plus quercetin (D+Q) belongs to a class of senotherapeutics called senolytics, which selectively kill senescent cells while sparing the non-senescent cells. D+Q was shown to prolong lifespan in animals and activate cell-death pathways within senescent cells. Given its positive safety profile in humans, D+Q was tested in 14 patients with idiopathic pulmonary fibrosis (a senescence-based condition). D+Q therapy significantly improved physical function and decreased inflammatory factors associated with aging.
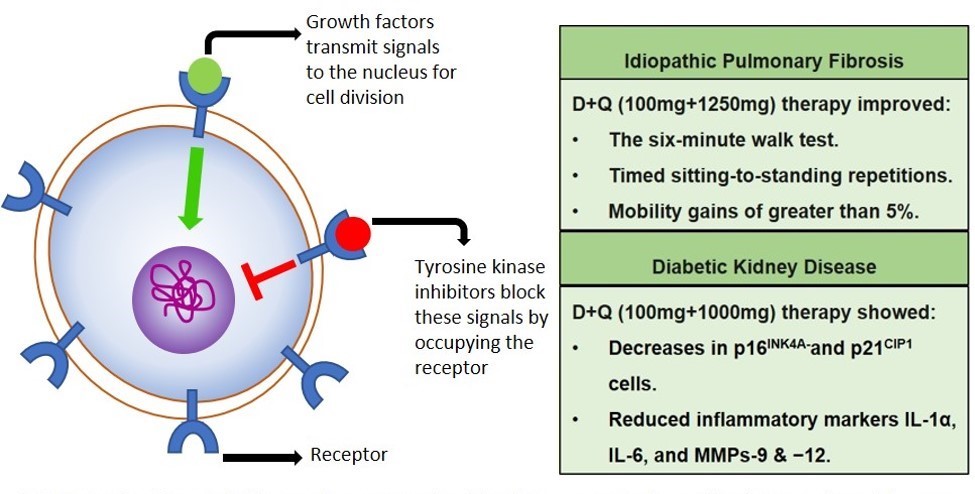
Dasatinib and quercetin (D+Q) blocks a protein called tyrosine kinase, thereby inducing cell death. Clinical trials in patients with idiopathic pulmonary fibrosis and diabetic kidney disease showed D+Q treatment resultsed in significant improvement in patient physical functioning.
Oxidative stress has been associated with several ARDs and is caused by high reactive oxygen species (ROS) levels, leading to DNA damage and cell senescence. ROS scavengers are antioxidants that show promise as effective anti-aging drugs. Resveratrol, a natural compound found in grapes, has been studied for its senotherapeutic properties. A study of patients with Parkinson's disease showed high ROS scavenging activity for vitamin-E-loaded resveratrol nanoemulsion. Levels of endogenous antioxidant enzymes like superoxide dismutase and glutathione peroxidase increased significantly, while levels of malondialdehyde, a biomarker for ROS activity, reduced significantly.
Another open-label study showed that resveratrol 5g daily for 12 weeks significantly reduced oxidative stress in patients suffering from Friedreich’s ataxia.
.jpg.aspx) Resveratrol is a natural molecule found in grapes. Resveratrol is an excellent ROS scavenger, preventing oxidative stress.
Resveratrol is a natural molecule found in grapes. Resveratrol is an excellent ROS scavenger, preventing oxidative stress.
Furthermore, administration of resveratrol 1g daily for 28 days was associated with high plasma antioxidant activity in healthy individuals. However, the critical issue of bioavailability limits the clinical use of resveratrol and similar polyphenols (research is in progress to address this issue).
Caloric restriction
Caloric restriction has been shown to increase rodent lifespan. In humans, cutting caloric intake by 15% for 2 years slowed aging and metabolism and protected against ARDs. It decreased systemic oxidative stress by decreasing ROS levels. However, this may not be a feasible approach since it is difficult for people to stick to a diet for a long period of time.
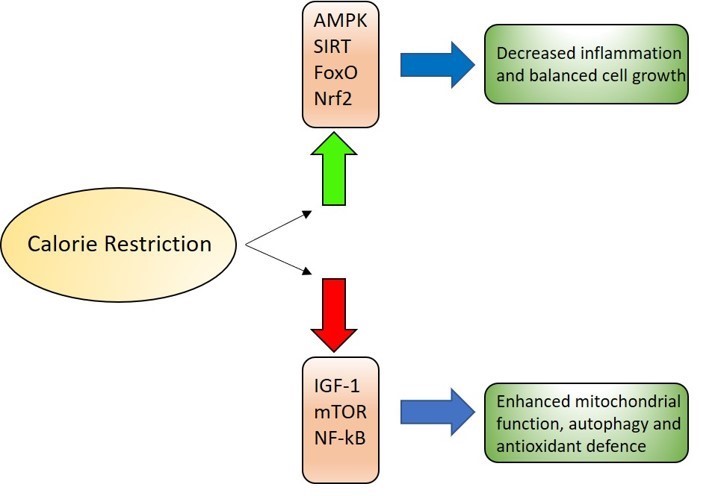
Caloric restriction activates the AMPK, sirtuin, FOXO and Nrf2 pathways while downregulating IGF-1 and mTOR pathways to prolong lifespan.
mTOR inhibition
Mechanistic target of rapamycin (mTOR) is a protein that has been implicated in cellular senescence. Therefore, blocking mTOR can lead to senotherapeutic effects. Rapamycin is an mTOR inhibitor used to prevent organ transplant rejection. However, because of its immunosuppressive side-effects, pharmaceutical companies have developed enhanced versions of rapamycin (rapamycin analogs) and other mTOR inhibitors. In animals, rapamycin has been shown to increase lifespan without side-effects. In humans, healthy, elderly patients showed no side-effects from oral rapamycin 1mg daily for 8 weeks and was well tolerated.
Patients treated with everolimus, another mTOR inhibitor, enhanced response to an influenza vaccine by about 20% which could help stave off at least one episode of respiratory infection. However, further large-scale studies are needed before it can be prescribed for ARDs.
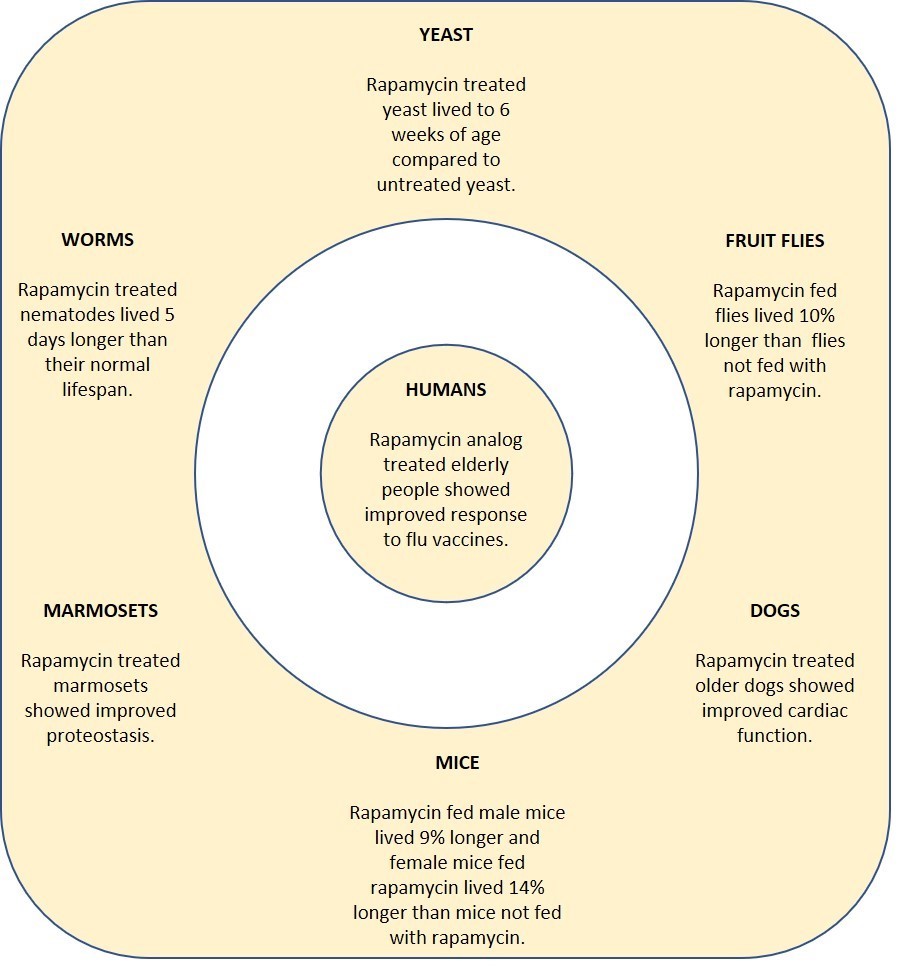 The anti-aging effects of rapamycin/rapamycin analogs were studied in yeast, worms, fruit files, ice, dogs and marmosets before being studied in humans (see text above for link to sources).
The anti-aging effects of rapamycin/rapamycin analogs were studied in yeast, worms, fruit files, ice, dogs and marmosets before being studied in humans (see text above for link to sources).
Metformin
Metformin is an anti-diabetic drug, popular because of its low cost and safety profile. It increases lifespan and health span in animals through its inhibitory effect on inflammation, oxidative stress, cellular senescence and cell death. The Metformin in Longevity Study (MILES) in humans suggests that metformin may reduce the occurrence of age‐related diseases like cardiovascular disease and cancer through its effect on metabolic genes, collagen and mitochondrial genes in adipose tissue, and DNA-repair genes in muscle. This study has also provided insight into the potential pathways involved in aging, which could be targets for novel anti-aging drug discovery.
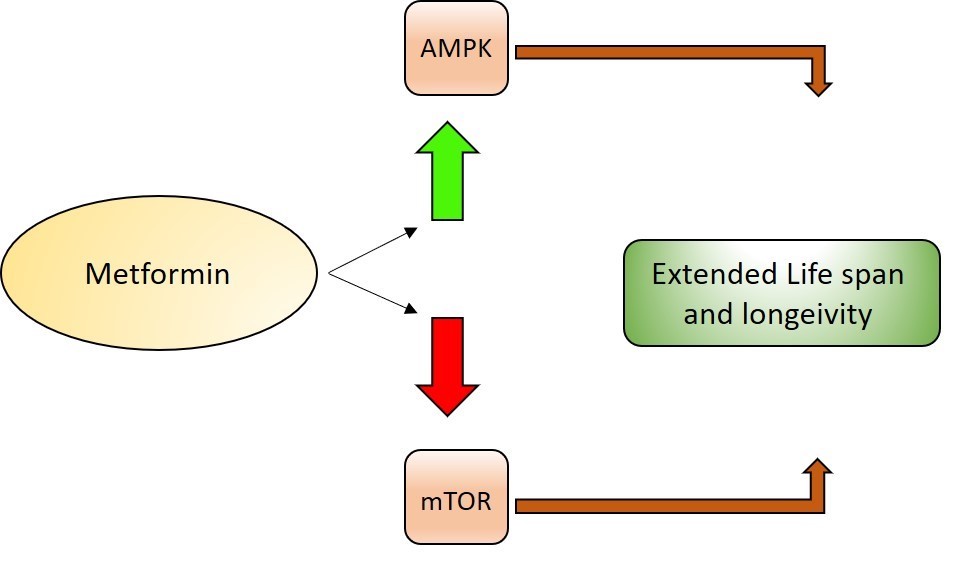
Metformin extends lifespan through AMP-activated protein kinase activation,which kickstarts autophagy to clear the cells of dysfunctional parts of the cell. It can also block mTOR to counter the effects of aging.
The future of aging
With so many potential compounds in the pipeline, senotherapy shows much promise. However, we must consider the consequences of interrupting a fundamental biological process and the challenges that may present. It is estimated that by 2100 over 25% of the population will be over 65, which will put considerable strain on global healthcare systems and economies if they are unhealthy. Therefore, it is crucial to make the concept of healthy aging a reality for our aging population.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.