The production of new therapeutics continues to be plagued by concerns of drug attrition during drug discovery and development. The development of more predictive tools of human physiology and toxicity to diminish attrition has been desired for decades. Simple human in vitro and animal models can be used in different contexts to assess drug suitability, but often they do not accurately predict the complex tissue or organ response from a patient.
An ideal pharmacological model would be directly relevant to human physiology, while maintaining complexity, be amenable to high-throughput screening, and overcome the ethical qualms of in vivo models.
Bioprinting – Printing the ideal research model
The rapidly developing technology of bioprinting could potentially meet these requirements. Bioprinting is the mechanical deposition of biological materials, controlled by a computerised model. There are various forms of bioprinting technology - from inkjet similar to household dye printers, extrusion-based, and laser-assisted. Controlling the precise deposition of biomaterials and cells in 3D allows fabrication of structures with tissue, and potentially even organ level complexity. Examples of various tissue types have already been produced, including cardiac, kidney, and liver. These can play a particularly important role in pharmacological and toxicological studies.
Recreating the tissue essentials
When considering design of bioprinted structures, it is important to consider the fundamental characteristics of biological tissue and what is currently lacking from currently used models. Tissue is 3D and harbours a precise 3D architecture where biological matrices and cell are positioned to interact with each other. Biomechanical properties are also specifically tuned in tissue, from shear stress, to rigidity. Alterations in these have striking effects on the biology of cells. Compare this to standard 2D in vitro culture in plastics and the depletion of complexity is striking. Bioprinting allows the production of more tissue-like structures than otherwise possible. By combining the various cell types and materials present in tissue into a close analogue of physiological tissue, drug development would be able to identify pharmacological effects only present in cell co-cultures, or in appropriately arranged or polarised cells.
Bioprinting with human cells and materials also provides a closer link to the potential patient than an animal model. Stem cells would give a renewable method of production and culture when compared to primary cell models. Incorporation of cells from a diverse pool of the population would also help improve predictive quality across varied genetic backgrounds of patients.
To be viable for high content screening, bioprinted products must be amenable to ‘scale up’. This is something relatively straightforward for bioprinting – as the process is automated and controlled by a computer model, the model and printing parameters can be tailored to alter produced unit size or complexity to fit the desired role of the model. The flexibility of bioprinting could allow printing of a large complex heart patch in the scale of centimetres, or the production of thousands of mini tissues on the micrometre scale.
Vascularisation – the holy grail of tissue engineering
The vasculature plays an unmistakable role in several diseases as well as pharmacological and toxicological research, and its absence from all current in vitro models speaks volumes to the poor predictive output we observe. Success has been achieved in producing bioprinted and microfluidic vasculature. It is possible to coax endothelial cells to form vascular lumina by addition of growth factors and mechanical flow through a microfluidic chamber. Bioprinting can utilise so-called ‘sacrificial gels’ in tubes, which melt out creating a hollow chamber for vascularisation. Unfortunately, so far it has not been possible to translate this into larger, complex embedded networks typically seen in tissue.
Organs-on-a-chip
Competing technology exists in the tissue engineering space in the form of “organs-on-a-chip” (OOCs). OOCs typically take the form of small microfluidic devices that harbour cell culture chambers in a bid to mimic the physical and physiological processes of tissues and organs. These also have many advantages over simple 2D and 3D in vitro models, including biomechanical properties and tissue-tissue interfaces. However, there are still drawbacks to this technique that give bioprinting an edge. OOC microfluidics often require encapsulation in plastics which can decrease scale up potential, and hamper analysis and characterisation of the cells. Complexity is also lost as true, embedded vascularisation seems difficult or impossible to reproduce in OOCs.
Current bioprinting in the market
Organovo is the current bioprinting industry leader that already offers human liver models for disease modelling and drug testing. Their product is based on primary human liver cells, including hepatocytes and supporting cells. Other companies such as BiogelX and CELLINK develop and provide the bioprinters and materials used for the printing process itself. Evidently, there is currently little market presence of bioprinted tissue products. This is likely to change as bioprinting technology matures and the sensitivity, specificity and predictive power for drug development of printed tissues improves.
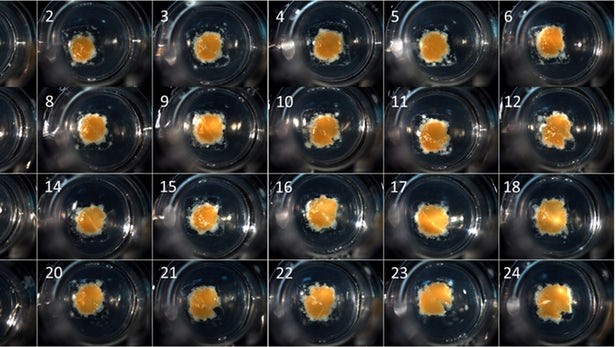 Organovo’s exVive3D 3D liver models, bioprinted and composed of hepatocytes and supporting cells. (Organovo)
Organovo’s exVive3D 3D liver models, bioprinted and composed of hepatocytes and supporting cells. (Organovo)
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.