Daniel Saffer is the winner of the 2024 ECP Writing Prize. Judges enjoyed his insight on osteoarthritis, and the future of treatment for this common condition.
Everyone knows somebody that has osteoarthritis (OA), whether of the hip, the knee, the digits, or the spine. In fact, it is so common that 1 in 5 adults over the age of 45 in England have osteoarthritis of the knee, and together with rheumatoid arthritis, will cost the NHS £118 billion over the next decade. Despite its prevalence and decades of research into the disease, there are still no effective treatments which modify the disease trajectory. Patients with osteoarthritis have their pain managed and will eventually have the joint replaced with an artificial one, which itself results in a long recovery period. There are, at present, no disease slowing or curative treatments for OA. Medicine is becoming ever-more sophisticated, yet management of this disease has not kept pace.
What causes OA itself is not entirely clear. It was originally thought of as a disease arising from general wear and tear, which is why age is a big risk factor. However, studies have shown that a person’s genetics do play a role in their susceptibility to the disease, as well as whether someone has had an injury to the area or if they are obese. In terms of what happens to the joint, the layer of cartilage that lines the bones becomes thinner, leading to general inflammation of the joint associated with pain. There are also changes to the bone itself, culminating in the person being less mobile, and the cells that make cartilage, chondrocytes, become unable to repair any damage.
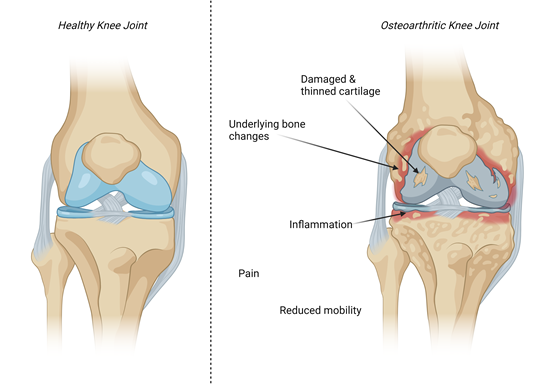
Figure 1: Pathophysiological changes in the osteoarthritic joint include damaged and thinned cartilage, subchondral bone changes and inflammation associated with pain and reduced mobility. Created with biorender.com.
At the molecular level, there are increased levels of inflammatory mediators called cytokines that propagate the disease and cause further destruction of cartilage. One such molecule, that was identified as early as the 1980s to be pro-inflammatory, is called tumour necrosis factor alpha (TNF-α). Research into TNF-α in the lab led scientists to believe that blocking the action of this molecule would prevent its action in progressing the disease.
Adalimumab, a monoclonal antibody that blocks TNF-α and is used to treat other auto-immune diseases such as Crohn’s disease, was found to not have any efficacy in preventing pain or improving mobility outcomes for both hand and knee osteoarthritis relative to placebo. It is given as an injection under the skin (subcutaneous). One possible explanation for this is that by the time a patient presents for treatment, the disease has progressed too far for this to be effective, which may not be the case if it was initiated in the early stages of the disease. This, however, would be impractical as it would require detecting the presence of disease before the onset of symptoms.
A similar strategy of trying to block the molecules that cause progression of OA was also tried with interleukin-1β blockers. Interleukin-1β is another pro-inflammatory molecule and has shown in lab studies that it causes further cartilage destruction over and above any wear and tear. In a clinical trial comparing knee OA patients receiving Anakinra (an interleukin-1β blocker) or a placebo, there was no improvement in OA symptoms when given as an injection into the joint space, called an intra-articular injection. This persistent failure of such trials could mean either that the drug itself is not efficacious (which we know not to be the case as) or as a scientific community, we have a serious lack of understanding about the disease. Moreover, it is thought that every patient with OA is different, and often only seek medical advice because of pain, which again, can be very variable. Subtypes of OA might mean that a treatment might work for one but not another, and there are efforts ongoing to stratify different groups of OA patients.
Nevertheless, not all is gloomy for patients with OA as research is continuing with some promising results. The most encouraging outcome of recent research is a drug called Sprifermin, which Merck is developing for use in OA. It is a laboratory-produced version of a natural protein found in the body, fibroblast-growth factor 18. It works to help chondrocytes regain their ability to repair the cartilage that has become damaged and was also delivered by intra-articular injection. In a clinical trial called FORWARD, Sprifermin was administered to 387 patients at two different doses either every 6 or every 12 months for a total of 18 months, and patients were followed up for a total of five years to assess long-term efficacy. Results showed that those taking Sprifermin had thicker knee cartilage and lower pain scores than those taking placebo. Both findings were also maintained at the five-year time point, which are encouraging results at this stage. The drug was also well tolerated, with low occurrence of side effects. The next step will be to do the next phase of trial, which will include much larger numbers of participants, and will home in on the most effective dose and dosing regimen for presentation to the regulators for approval.
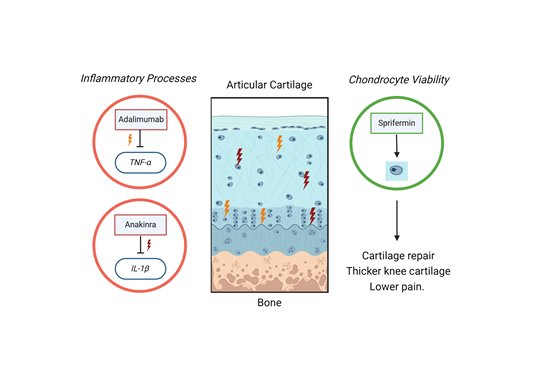
Figure 2: Strategies for treating OA have targeted inflammatory processes unsuccessfully. Treatments targeted towards increased chondrocyte viability and ability to repair the cartilage tissue have been more successful, leading to lower pain scores and thicker knee cartilage. Created with biorender.com.
As such, it could be argued from a treatment perspective that the progression of OA is more heavily influenced by the inability of cartilage cells to repair damage (in conjunction with other factors), rather than the inflammatory processes. Treatment with Sprifermin is also promising because whilst an injection into the knee might be unpleasant, it only needs to occur once or twice a year and its effects are maintained for many years post treatment. This would also confer significant savings when compared to inpatient surgery to replace the affected joint. Even if Sprifermin does not make it through regulatory approval, it has been shown that this strategy of treating OA can be effective and can be exploited through other avenues.
It seems that the case of OA is another example of where findings from the lab are not translated through animal models to eventual human benefit. Whilst strides have been made in the surgical management of OA in the past few decades, there has not been the same development in pharmacological treatment. Thanks to the continued dedication of musculoskeletal researchers, this may not be the case for much longer to the benefit of society, the healthcare systems and OA patients themselves.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.