Inflammation, a natural immune response to harmful stimuli, is associated with various diseases such as cardiovascular disease, cerebrovascular conditions, diabetes, obesity, autoimmune disorders, and certain cancers when this initially beneficial response is not properly regulated. Tackling the ever-growing chronic inflammatory burden and the associated impact on healthcare resources in aging Western societies is becoming increasingly relevant. Novel therapeutic options and new drug targets are urgently needed. The G protein-coupled pattern recognition receptors termed Formyl Peptide Receptors (FPRs), might help with this task.
The impact of FPR research was highlighted during two dedicated sessions at the 19th World Congress of Basic & Clinical Pharmacology 2023 (WCP2023) in Glasgow. Session 1 ‘
Resolution Pharmacology – new therapeutic approaches for the treatment of inflammatory diseases’ was chaired by Patrícia Silva, who is a researcher in Public Health, Oswaldo Cruz Foundation, Rio de Janeiro (Brazil), and Mauro Perretti, who is a Professor of Immunopharmacology at Queen Mary University of London (UK). This session was dedicated to the advanced approaches to target FPR2 to counteract inflammatory processes and the development of FPR2-directed drugs to promote the resolution of inflammation.
FPR2 arose from the ancestral FPR1 and the
shared sequence identities of approximately 70% indicate a close functional relationship between the two receptors. Notably, the agonist binding sites of FPR1 and FPR2 display an even higher degree of
similarity of 89%. Both receptors couple to the inhibitory alpha (i) G protein subfamily for
signal transduction. Most
agonists interact with both FPRs yet often differ in the associated logistic parameters. To date, only very few selective ligands are known. Both
FPRs are predominantly expressed in neutrophils and macrophages. However, apart from cells of the myeloid lineage, both receptors are detected in various non-myeloid tissues. While FPR1 is most often linked with proinflammatory signaling, FPR2 is associated with the resolution of inflammation (see figure1 and hyperlink). The anti-inflammatory and pro-resolving effects of FPR2 activation have been effectively demonstrated through the use of annexin A1, a protein that is abundantly found in neutrophils, and Ac2-26, its N-terminal cleavage product which retains the FPR binding site. Based on the promising results obtained with this FPR2 peptide ligand, derivatives are currently progressing toward clinical application in
resolution therapy.
The striking FPR1/FPR2 similarities and the resulting implications were explored in depth in the second WCP2023 session “
Yin and Yang of Formyl peptide receptors", which was chaired by Felicity Gavins, Adriano Rossi and Ursula Rescher; who are Professors from Brunel University London (UK), the University of Edinburgh (UK) and the University of Münster (Germany), respectively. Here, the major focus was on the detailed analysis of the signalling repertoire of both receptors and their comparison utilizing comprehensive sets of ligands for the identification of receptor- and/or agonist-specific cellular responses. The underlying theme here was “
biased signaling”, an important concept in GPCR research for the development of drugs that specifically activate the desired pathways out of the entire signalling repertoire that is associated with a defined receptor. Molecular modelling will help establish
pharmacophores and as such the design of peptidomimetics and small molecules that potentially serve as lead structures for future drugs with
tailored responses.
During this session, a surprising picture emerged: FPR1 and FPR2 are not linked to exclusive and receptor-specific signalling profiles that would explain opposing functional patterns. Instead, only quantitative ligand-specific differences prevailed: The first FPR2 pro-resolving peptide agonist Ac2-26 initiated MAP kinase activation at both receptors, however, a reliable inhibition of
de novo cAMP generation was only observed at FPR1. Potentially, a similar
FPR1 activation profile thus might lead to the same desired outcome?
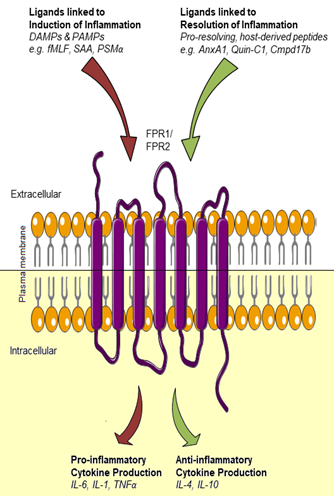 Figure 1: Dual outcomes of FPR activation. Upon receptor binding, ligands mediate either pro-inflammatory or pro-resolving responses, evidenced by the corresponding cytokine release profiles. Ligand-stabilized active conformational states might be receptor- or ligand-encoded, thus explaining the opposing effects. FPR1 and FPR2 share a vast spectrum of ligands. Both receptors most likely also share a core signalling profile, and the ligand-induced pro-inflammatory or anti-inflammatory effects thus might depend on the precise nature of the ligand-receptor interaction.
Figure 1: Dual outcomes of FPR activation. Upon receptor binding, ligands mediate either pro-inflammatory or pro-resolving responses, evidenced by the corresponding cytokine release profiles. Ligand-stabilized active conformational states might be receptor- or ligand-encoded, thus explaining the opposing effects. FPR1 and FPR2 share a vast spectrum of ligands. Both receptors most likely also share a core signalling profile, and the ligand-induced pro-inflammatory or anti-inflammatory effects thus might depend on the precise nature of the ligand-receptor interaction.
WCP2023 impressively expanded the understanding of FPR biology in two ways. While reconfirming the functional relevance of FPR2 signalling towards approaching clinical use, the systematic comparison of FPR1 and FPR2 signalling profiles points to a shift in perspective regarding the opposing receptor functions in inflammation. These research avenues may lead to the addition of FPR1 as a potential drug target.