_1.png.aspx)
Semaglutide, more widely known under its brand name Ozempic, received authorisation for use in the EU in March 2018. Despite the original indication to improve glycaemic control in adults with type II diabetes (T2D), evidence began to emerge that Ozempic aided with weight loss in patients receiving the drug. In 2021, the Food and Drug Administration (FDA) approved a formulation with a higher dose of semaglutide (Wegovy) to treat obesity.
This sparked a wave of celebrities, influencers, and even well-known businessman Elon Musk, praising semaglutide for facilitating weight loss in short time frames. Testament to the influence and power of social media, Ozempic quickly gained popularity, leading to ongoing global shortages and concern around the dangers of off-label prescribing and uneducated self-administration.
Mechanism of action
Briefly, Ozempic acts as a GLP-1 receptor agonist, selectively binding and activating the GLP-1 receptor, found abundantly in pancreatic β-cells. This stimulates insulin secretion and lowers glucagon secretion in a glucose-dependent manner, thus lowering fasting and post-prandial blood glucose and improving glycaemic control. Ozempic also delays gastric emptying. Whilst this slows the rate that glucose appears in the circulation, it is also thought that this slower movement of food through the gut extends the feeling of fullness post-meal, and also reduces appetite. It is these effects that are thought to contribute to the weight loss observed with GLP-1 agonists.
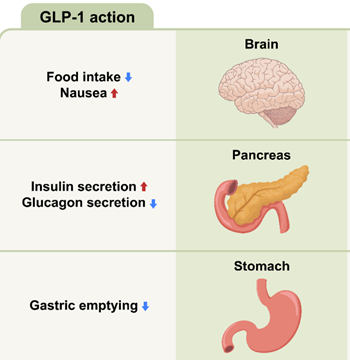
Adapted from Roh and Choi (2023)
Social impact of Ozempic
The number of Ozempic prescriptions in the United States has grown by more than 5,000% since 2018, up to around 20 million in 2023. Even notable weight loss programmes like WeightWatchers are now prescribing weight loss medications. Whilst accessibility to healthcare and pharmaceuticals is important, the large amount of misinformation available online is potentially harmful. Side effects of Ozempic are usually mild; however more severe side effects, including pancreatitis and stomach paralysis, have been recorded. It is therefore important that patients receiving Ozempic are regularly monitored by a medical professional.
However, as demand grows, so do the numbers of people receiving GLP-1 agonist drugs from sources with little or no follow-up care. This could lead to inappropriate dosing, inadequate monitoring of side effects, and potential interactions with other medications, posing risks to individual health and safety. However, due to the ever-growing societal pressure to live up to unachievable and unrealistic body standards, coupled with the potentially unethical promotion of weight loss medication, it is likely that these drugs will continue to gain popularity and garner impressive profits. This raises questions on how we can monitor misinformation and irresponsible marketing online to ensure weight loss medication is prescribed and taken correctly.
Ozempic in public health
Obesity is a growing public health epidemic that has been found to increase the risk of T2D, cardiovascular disease and certain types of cancer. In 2021, a quarter of adults in the UK were found to be obese. Currently, Wegovy is offered as an option for weight management on the NHS if the patient has at least one weight related comorbidity (i.e. high BMI, >30 kg/m2), in conjunction with specialist weight management service and agreement to provide the drug. However, the allure of Wegovy and its societal profile raises the question of the future of obesity management, as patients with access to private care may be able to obtain it without meeting the National Institute for Health and Care Excellence (NICE) requirements.
Tackling obesity has focused on changing dietary habits to increasing the portion of daily intake of fruit, vegetables, legumes and engaging in regular physical exercise on a weekly basis. The emphasis on maintaining a healthy lifestyle can be challenging for individuals, even with support from healthcare professionals. Therefore, anti-obesity medication that has minimal side effects could be seen as a shortcut to maintaining healthy weight. The recent FDA approval of Wegovy to treat cardiovascular risk in overweight and obese adults, its use has been touted to advance public health management of obesity and cardiovascular disease. That said, as the intended use is for overweight and obese adults, Wegovy’s use by the broader public for weight management may lead to unintended consequences.
Looking to the future
While the successes of Ozempic are making headlines, its ‘celebrity’ profile as a low-risk, anti-obesity medication has surged its demand. This in turn is causing shortages as people scramble to secure the drug. As patients with T2D are caught without Ozempic, its popularity may be detrimental to treating individuals as the supply chain is adjusting to increasing demand. The silver lining is that there are multiple treatment options for patients with T2D, so the current limit to accessing Ozempic may not severely impact their health outcome. Regardless, Ozempic’s impact on society and public health management of obesity has caused an influx of additional anti-obesity therapeutic alternatives. This, in turn, may be the turning point for tackling the obesity epidemic and impact future incidence of T2D.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.