A little over a year ago, the UK confirmed its first case of COVID-19. Since then, we have collectively endured months of uncertainty and lockdowns whilst scientists worldwide endeavoured to create a vaccine. This process normally takes upwards of 10 years, but due to extraordinary cooperation between countries, scientists, medical professionals and regulatory boards, the UK has so far approved three coronavirus vaccines (Pfizer/BioNTech, Oxford University/AstraZeneca, and Moderna) for emergency use as of 1 February 2021.
What types of vaccine are there for COVID-19?
Traditional vaccines
More ‘traditional’ vaccines contain a dead or disabled virus, which is designed to provoke an immune response that provides protection against the live virus, but is incapable of causing severe disease. Live-attenuated vaccines use a weakened virus and inactivated vaccines use 'dead' viruses ('inactivated' is used because some scientists do not consider viruses to be alive). While these approaches are well-used and provoke robust and long-lasting immune responses, there are several drawbacks. For a live-attenuated virus, an obvious safety concern is that the virus might gain genetic changes that enable it to revert to more virulent strain. Another worry is that a mistake during manufacturing could produce a defective vaccine and cause a disease outbreak, which once happened with a polio vaccine. For inactivated vaccines, there is a risk of vaccine-enhanced disease.
mRNA vaccines
mRNA vaccines are unlike more ‘traditional’ vaccines as they do not contain the virus at all. In the case of the coronavirus vaccines, lipid nanoparticles (LNPs) encase mRNA which encodes the spike protein of the virus. Once the LNPs get the RNA into the cell, rapid production of the protein (antigen) occurs in the cytoplasm. The antigen, in this case the spike protein, is displayed to the immune system by the cells to teach it to mount a response. Once the protein is made, the instructions are no longer needed so the mRNA is degraded. Of the three UK approved vaccines, two are mRNA vaccines. Moderna fast‐tracked their candidate vaccine mRNA‐1273 and were first to begin clinical trials on 17 March 2020 and had their vaccine approved on 8 January 2021. Pfizer and BioNTech collaborated to create a similar vaccine which was the first of any to be approved on 2 December 2020 and deployed on 8 December 2020.
Viral vector vaccines
The University of Oxford/AstraZeneca vaccine uses a different strategy – a live recombinant viral vector. Using a virus which causes the common cold in chimpanzees as a Trojan horse, the Oxford vaccine smuggles the coronavirus gene into human cells to make the spike protein. The adenovirus vector is modified so it does not cause disease in humans and cannot replicate and has a gene from SARS-CoV-2 added in. This vaccine was approved on 30 December 2020 and was deployed on 4 January 2021.
Still in trials
In addition to these approved vaccines, there are many vaccines in different stages of clinical trials. Imperial College London and its spin‐out social enterprise, VacEquity Global Health, are developing an saRNA vaccine that works in a similar way to the mRNA vaccines but requires a lower dose as it contains a replicase enzyme allowing it to amplify itself within the cells. The Novavax vaccine is a protein-based vaccine combining an engineered protein from the virus with a saponin-matrix based adjuvant to generate a stronger immune response. The Johnson and Johnson vaccine, similarly to Oxford/AstraZeneca approach, uses a viral vector. With so many vaccines in development, this is clearly a fast-moving space. The different kinds of vaccine are summarised in Figure 1, with up-to-date information accessible from the London School of Hygiene & Tropical Medicine here and from the WHO vaccine tracker here.
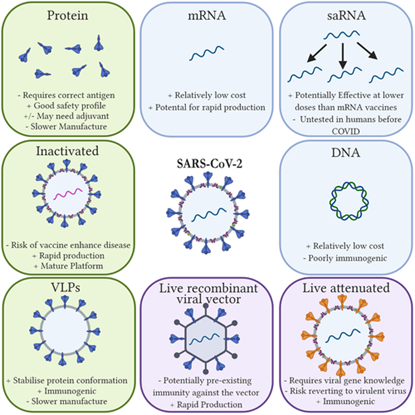
Figure 1. Platforms in use or in development for SARS-CoV-2 vaccines, taken from the paper Vaccines for COVID-19, with permission. Abbreviations: mRNA = messenger RNA, saRNA = self-amplifying RNA, VLP = virus-like particle.
How are the vaccines similar and different?
All vaccines share the same end goal – to provide protection against COVID-19 infection. WHO recommends that successful vaccines should show disease risk reduction of at least 50%, with 95% CI that true vaccine efficacy exceeds 30%. There are similarities and differences in platform, manufacturing, distribution and efficacy between the vaccines, but all so far provide suitable levels of protection (Table 1).
| Vaccine |
Approval |
Type |
Manufacture |
Distribution |
Efficacy |
| Pfizer/BioNTech |
UK, EU, US |
mRNA |
Cultured cell materials |
-70°C |
95% |
| Oxford/ AstraZeneca |
UK, EU |
Viral vector |
Grown from cells infected with virus then filtered and purified |
Up to 6 months in a normal 2-8°C refrigerator |
60-90% |
| Moderna |
UK, EU, US |
mRNA |
Cultured cell materials |
2-8°C for 30 days and -20°C for up to 6 months
|
95% |
| Novavax |
Phase III ended, not yet approved |
Protein subunit |
Spike proteins produced by moth cells infected with genetically modified version of coronavirus. Also contains an “adjuvant”, which enhances immune response |
2-8°C |
89.3% |
| Johnson and Johnson |
Phase III ended, not yet approved |
Viral Vector |
Grown from cells infected with virus then filtered and purified |
2-8°C |
85% |
Table 1. Summary of COVID-19 vaccine design, manufacture and efficacy.
One of the main differences between the vaccines is how they must be stored, and this impacts the ease of distribution, especially to less affluent countries (Table 1). The Oxford/AstraZeneca and Moderna vaccines are much easier to store and distribute than the BioNTech/Pfizer vaccine. The Moderna vaccine can be stored in a fridge for 30 days and in a normal household or medical freezer (-20°C) for up to 6 months and requires no dilution prior to use. The Oxford vaccine is transported to vaccination centres in refrigerated vans (between 2 and 8°C). In contrast, the Pfizer vaccine must be kept at around -80°C to maintain optimal activity and requires mixing with another liquid prior to administration. As a result, Pfizer developed its own packaging, and the doses must be flown in from Belgium and sent to vaccination centres in GPS-tracked trucks with thermo sensors. Recent data submitted to the FDA however, has shown that the vaccine remains stable after storage of the undiluted vials for up to two weeks at standard freezer temperature. This is not applicable to the storage of thawed vials before dilution (which can be held in the refrigerator for up to 5 days), or on the storage of thawed vials after dilution (which can be held at refrigerator temperature or room temperature for use within 6 hours).
Efficacy – what does it mean for the vaccines?
Before directly comparing each of the vaccines, it is important to remember that the trials all use different criteria for what counts as infection, and this can lead to variations in results regarding efficacy. Various endpoints are used to define efficacy depending on the pathogen, transmission dynamics and consequences of infection. Often, the data from trials are presented as a proportional reduction in disease between vaccinated and control participants, to calculate the reduction attributable to the vaccine. Outcomes tend to include reduction in infection (i.e., sterilising immunity), disease severity, or duration of infectiousness. However, these trials represent the best-case scenario of efficacy under idealised conditions within chosen populations and provide the data needed for licence. This does not always accurately predict real-world efficacy. These trials might not predict indirect protection gained from herd immunity, or differences in immunity related to age, ethnicity, sex, comorbidities, or vaccine hesitance. For this reason, prospective studies of vaccine effectiveness in real-world scenarios post-licensing are routinely needed. Despite these caveats, in the case of SARS-CoV-2, an efficacious vaccine might prevent infection, disease, or transmission and every vaccine will reduce hospitalisation and deaths.
Variants, escape and vaccines
A new virus variant has one or more mutations differentiating it from the wild-type or predominant virus variants circulating among the general population. As expected, over 4,000 SARS-CoV-2 variants have been identified across the world. In the past few months, several new variants of concern have been announced. The focus here will be placed on the Kent (B.1.1.7), Brazil (P.1) and South African (B.1.351) strains. One specific mutation, called D614G, is shared by these three variants (Table 2). There also is epidemiologic evidence that variants with this specific mutation spread more quickly than viruses without the mutation, increasing transmissibility. In addition, variants cause disparities in vaccine efficacy as outlined in Table 2, therefore much work is in progress to ensure people remain protected. Making sense of how the variants continue to impact the pandemic falls to the newly formed G2P-UK National Virology Consortium. Its remit is to use cell cultures and animal models to examine how the mutations affect the transmissibility of the virus, the severity of the disease, and the effectiveness of the vaccines and treatments. The initiative brings together researchers across the UK, who will work alongside the COVID-19 Genomics UK Consortium to ensure that the vaccines continue to provide protection as new variants emerge.
| Variant |
First Detected |
Key Mutations |
Transmissibility Rate |
Vaccine Efficacy |
| B.1.1.7 |
UK |
- 69/70 deletion
- 144Y deletion
- N501Y
- A570D
- D614G
- P681H
|
~50% increase in comparison to previously circulating strains |
Oxford/AZ |
Pfizer |
Moderna |
| 74.6% |
No evidence of reduction |
Unknown but reports of decreased neutralising antibodies |
| P.1 |
Japan/
Brazil |
- E484K
- K417N/T
- N501Y
- D614G
|
1.4-2.2 times more transmissible |
Unknown |
Unknown |
Unknown |
| B.1.351 |
South Africa |
|
Increased, but no specific numbers yet |
TBC (but some reports being as low as 10%) |
Unknown |
Reduced neutralising antibodies |
Table 2. Summary of three key variants of concern.
SARS-CoV-2 has caused a pandemic which, owing to its very high transmission rate, has affected nearly every corner of the world in the last year. Since the presentation of the disease is heterogenous, the development of vaccines is crucial to controlling the disease. While COVID-19 has highlighted gaps in public health preparedness, worldwide cooperation at every step of the process has culminated in over 200 vaccines so far, with several being licenced for public use. Despite the obvious fallout of a pandemic, one positive is that we as scientists have shown that open collaboration on a global scale is indeed possible and that by working together, we can solve even the most challenging of problems.
Comments
If you are a British Pharmacological Society member, please
sign in to post comments.